Using Flow Cytometry to Detect Apoptosis in Lung
- Slides: 1

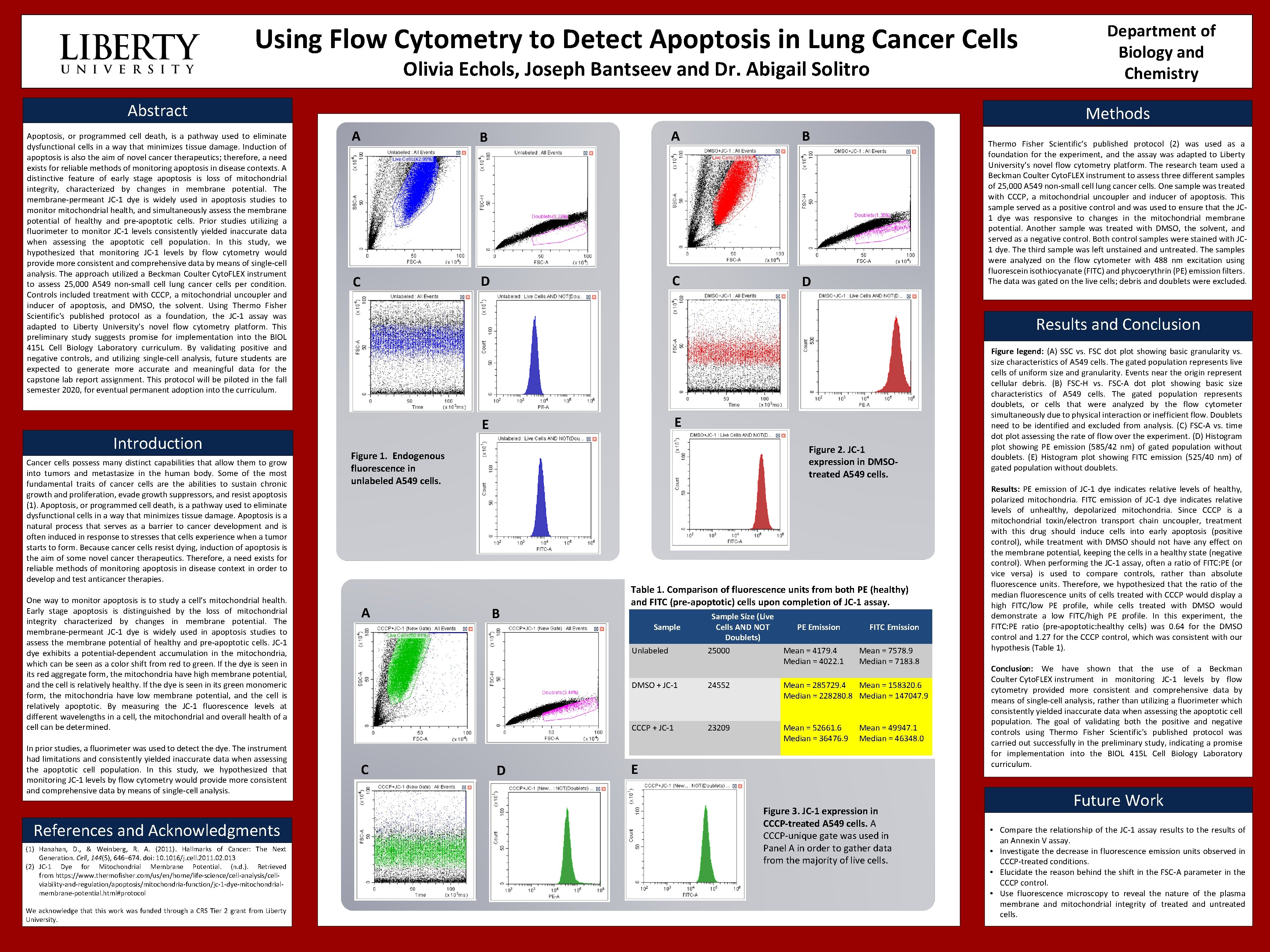
Using Flow Cytometry to Detect Apoptosis in Lung Cancer Cells Olivia Echols, Joseph Bantseev and Dr. Abigail Solitro Abstract Methods Apoptosis, or programmed cell death, is a pathway used to eliminate dysfunctional cells in a way that minimizes tissue damage. Induction of apoptosis is also the aim of novel cancer therapeutics; therefore, a need exists for reliable methods of monitoring apoptosis in disease contexts. A distinctive feature of early stage apoptosis is loss of mitochondrial integrity, characterized by changes in membrane potential. The membrane-permeant JC-1 dye is widely used in apoptosis studies to monitor mitochondrial health, and simultaneously assess the membrane potential of healthy and pre-apoptotic cells. Prior studies utilizing a fluorimeter to monitor JC-1 levels consistently yielded inaccurate data when assessing the apoptotic cell population. In this study, we hypothesized that monitoring JC-1 levels by flow cytometry would provide more consistent and comprehensive data by means of single-cell analysis. The approach utilized a Beckman Coulter Cyto. FLEX instrument to assess 25, 000 A 549 non-small cell lung cancer cells per condition. Controls included treatment with CCCP, a mitochondrial uncoupler and inducer of apoptosis, and DMSO, the solvent. Using Thermo Fisher Scientific's published protocol as a foundation, the JC-1 assay was adapted to Liberty University’s novel flow cytometry platform. This preliminary study suggests promise for implementation into the BIOL 415 L Cell Biology Laboratory curriculum. By validating positive and negative controls, and utilizing single-cell analysis, future students are expected to generate more accurate and meaningful data for the capstone lab report assignment. This protocol will be piloted in the fall semester 2020, for eventual permanent adoption into the curriculum. References and Acknowledgments (1) Hanahan, D. , & Weinberg, R. A. (2011). Hallmarks of Cancer: The Next Generation. Cell, 144(5), 646– 674. doi: 10. 1016/j. cell. 2011. 02. 013 (2) JC-1 Dye for Mitochondrial Membrane Potential. (n. d. ). Retrieved from https: //www. thermofisher. com/us/en/home/life-science/cell-analysis/cellviability-and-regulation/apoptosis/mitochondria-function/jc-1 -dye-mitochondrialmembrane-potential. html#protocol We acknowledge that this work was funded through a CRS Tier 2 grant from Liberty University. B A B C D Thermo Fisher Scientific’s published protocol (2) was used as a foundation for the experiment, and the assay was adapted to Liberty University’s novel flow cytometry platform. The research team used a Beckman Coulter Cyto. FLEX instrument to assess three different samples of 25, 000 A 549 non-small cell lung cancer cells. One sample was treated with CCCP, a mitochondrial uncoupler and inducer of apoptosis. This sample served as a positive control and was used to ensure that the JC 1 dye was responsive to changes in the mitochondrial membrane potential. Another sample was treated with DMSO, the solvent, and served as a negative control. Both control samples were stained with JC 1 dye. The third sample was left unstained and untreated. The samples were analyzed on the flow cytometer with 488 nm excitation using fluorescein isothiocyanate (FITC) and phycoerythrin (PE) emission filters. The data was gated on the live cells; debris and doublets were excluded. E E Figure 2. JC-1 expression in DMSOtreated A 549 cells. Figure 1. Endogenous fluorescence in unlabeled A 549 cells. Cancer cells possess many distinct capabilities that allow them to grow into tumors and metastasize in the human body. Some of the most fundamental traits of cancer cells are the abilities to sustain chronic growth and proliferation, evade growth suppressors, and resist apoptosis (1). Apoptosis, or programmed cell death, is a pathway used to eliminate dysfunctional cells in a way that minimizes tissue damage. Apoptosis is a natural process that serves as a barrier to cancer development and is often induced in response to stresses that cells experience when a tumor starts to form. Because cancer cells resist dying, induction of apoptosis is the aim of some novel cancer therapeutics. Therefore, a need exists for reliable methods of monitoring apoptosis in disease context in order to develop and test anticancer therapies. In prior studies, a fluorimeter was used to detect the dye. The instrument had limitations and consistently yielded inaccurate data when assessing the apoptotic cell population. In this study, we hypothesized that monitoring JC-1 levels by flow cytometry would provide more consistent and comprehensive data by means of single-cell analysis. A Results and Conclusion Introduction One way to monitor apoptosis is to study a cell’s mitochondrial health. Early stage apoptosis is distinguished by the loss of mitochondrial integrity characterized by changes in membrane potential. The membrane-permeant JC-1 dye is widely used in apoptosis studies to assess the membrane potential of healthy and pre-apoptotic cells. JC-1 dye exhibits a potential-dependent accumulation in the mitochondria, which can be seen as a color shift from red to green. If the dye is seen in its red aggregate form, the mitochondria have high membrane potential, and the cell is relatively healthy. If the dye is seen in its green monomeric form, the mitochondria have low membrane potential, and the cell is relatively apoptotic. By measuring the JC-1 fluorescence levels at different wavelengths in a cell, the mitochondrial and overall health of a cell can be determined. Department of Biology and Chemistry A B Table 1. Comparison of fluorescence units from both PE (healthy) and FITC (pre-apoptotic) cells upon completion of JC-1 assay. Sample Unlabeled Sample Size (Live Cells AND NOT Doublets) 25000 1 A C 2 PE Emission Mean = 4179. 4 3 Median 4 5 6= 4022. 1 7 8 9 FITC Emission Mean = 7578. 9 10 Median 11 12 13= 7183. 8 14 DMSO + JC-1 24552 Mean = 285729. 4 Mean = 158320. 6 Median = 228280. 8 Median = 147047. 9 CCCP + JC-1 23209 Mean = 52661. 6 Median = 36476. 9 B C D Mean = 49947. 1 Median = 46348. 0 E Figure 3. JC-1 expression in CCCP-treated A 549 cells. A CCCP-unique gate was used in Panel A in order to gather data from the majority of live cells. Figure legend: (A) SSC vs. FSC dot plot showing basic granularity vs. size characteristics of A 549 cells. The gated population represents live cells of uniform size and granularity. Events near the origin represent cellular debris. (B) FSC-H vs. FSC-A dot plot showing basic size characteristics of A 549 cells. The gated population represents doublets, or cells that were analyzed by the flow cytometer simultaneously due to physical interaction or inefficient flow. Doublets need to be identified and excluded from analysis. (C) FSC-A vs. time dot plot assessing the rate of flow over the experiment. (D) Histogram plot showing PE emission (585/42 nm) of gated population without doublets. (E) Histogram plot showing FITC emission (525/40 nm) of gated population without doublets. Results: PE emission of JC-1 dye indicates relative levels of healthy, polarized mitochondria. FITC emission of JC-1 dye indicates relative levels of unhealthy, depolarized mitochondria. Since CCCP is a mitochondrial toxin/electron transport chain uncoupler, treatment with this drug should induce cells into early apoptosis (positive control), while treatment with DMSO should not have any effect on the membrane potential, keeping the cells in a healthy state (negative control). When performing the JC-1 assay, often a ratio of FITC: PE (or vice versa) is used to compare controls, rather than absolute fluorescence units. Therefore, we hypothesized that the ratio of the median fluorescence units of cells treated with CCCP would display a high FITC/low PE profile, while cells treated with DMSO would demonstrate a low FITC/high PE profile. In this experiment, the FITC: PE ratio (pre-apoptotic: healthy cells) was 0. 64 for the DMSO control and 1. 27 for the CCCP control, which was consistent with our hypothesis (Table 1). Conclusion: We have shown that the use of a Beckman Coulter Cyto. FLEX instrument in monitoring JC-1 levels by flow cytometry provided more consistent and comprehensive data by means of single-cell analysis, rather than utilizing a fluorimeter which consistently yielded inaccurate data when assessing the apoptotic cell population. The goal of validating both the positive and negative controls using Thermo Fisher Scientific's published protocol was carried out successfully in the preliminary study, indicating a promise for implementation into the BIOL 415 L Cell Biology Laboratory curriculum. Future Work • Compare the relationship of the JC-1 assay results to the results of an Annexin V assay. • Investigate the decrease in fluorescence emission units observed in CCCP-treated conditions. • Elucidate the reason behind the shift in the FSC-A parameter in the CCCP control. • Use fluorescence microscopy to reveal the nature of the plasma membrane and mitochondrial integrity of treated and untreated cells.