Using Energy and Heat LESSON 3 PARTICLES IN

- Slides: 11

Using Energy and Heat LESSON 3 PARTICLES IN MOTION

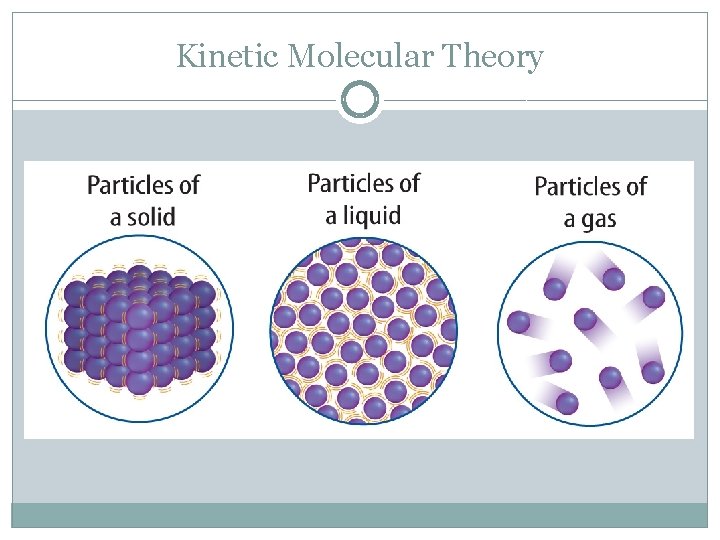
Kinetic Molecular Theory �Scientific theory that explains how particles move. � 3 main points: All matter is made of particles (atoms) Particles are in constant, random motion Particles constantly collide with each other and with the walls of their containers � 3 main types of matter: Solid (particles move slowest) Liquid (particles move faster than solid) Gas (particles move fastest)

Kinetic Molecular Theory

Kinetic Molecular Theory �Temperature – the measure of the average kinetic energy of the particles in a material. Particles that move slower (like in solids) occupy less volume than they do at higher temperatures. Thermal expansion – increase in volume of a material due to a temperature increase. � Except for water! When water freezes, interactions between water molecules push the molecules apart. � This is why ice floats on water (because it is less dense than the liquid).

Kinetic Molecular Theory �As the kinetic energy of the particles that make up an object increases, thermal energy increases The more they move, the hotter the object gets The less they move, the colder the object gets

Kinetic Molecular Theory

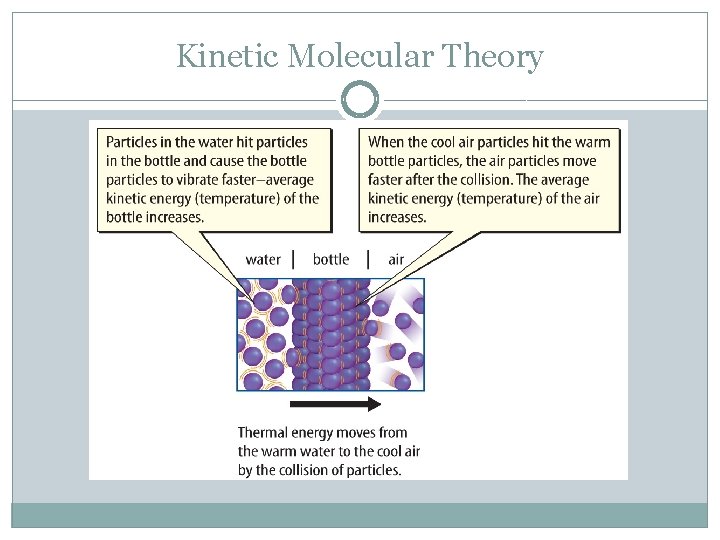
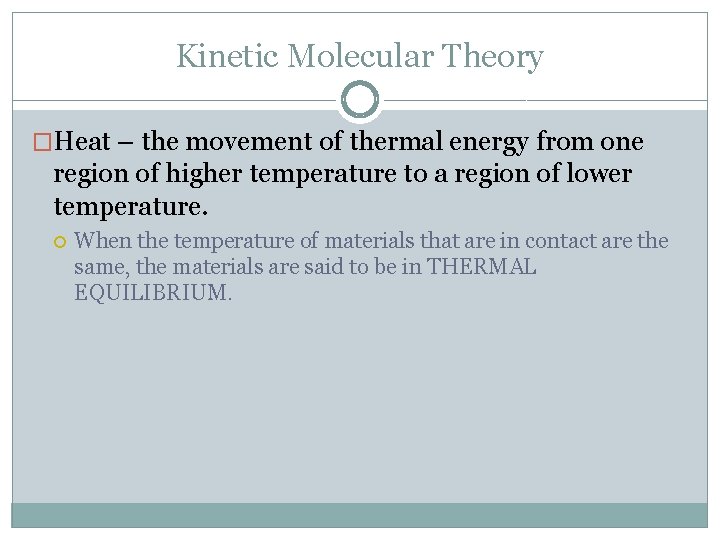
Kinetic Molecular Theory �Heat – the movement of thermal energy from one region of higher temperature to a region of lower temperature. When the temperature of materials that are in contact are the same, the materials are said to be in THERMAL EQUILIBRIUM.

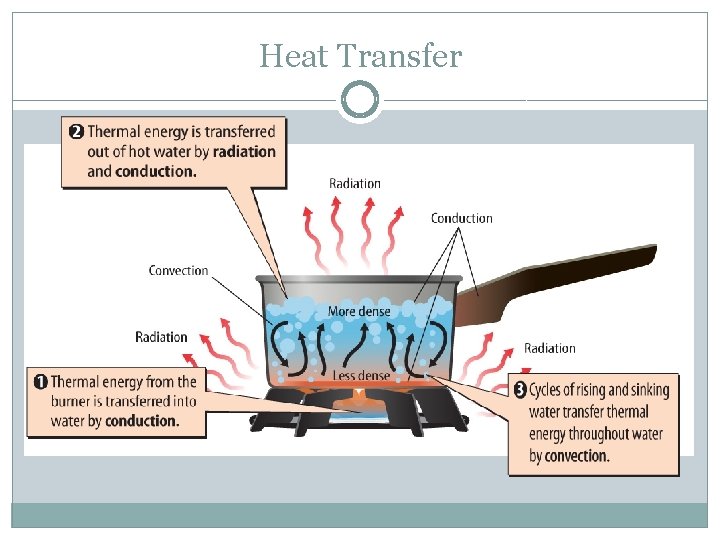
Heat Transfer �Conduction – the transfer of thermal energy by collisions between particles in matter �Radiation – the transfer of thermal energy by electromagnetic waves �Convection – the transfer of thermal energy by the movement of the particles from one part of a material to another

Heat Transfer

Changes of State �Melting = solid to liquid �Freezing = liquid to solid �Vaporization = liquid to gas Boiling = vaporization inside the liquid Evaporation = vaporization at the surface of the liquid �Sublimation = solid changes to gas without going through a liquid stage �Deposition = gas changes to solid without going through a liquid stage Water vapor goes through deposition when it freezes to frost

Conductors and Insulators �Thermal conductor – material in which thermal energy moves quickly �Thermal insulator – material in which thermal energy moves slowly