Using Concentrations of 3 Solutions in moldm Describe

Using Concentrations of 3 Solutions in mol/dm
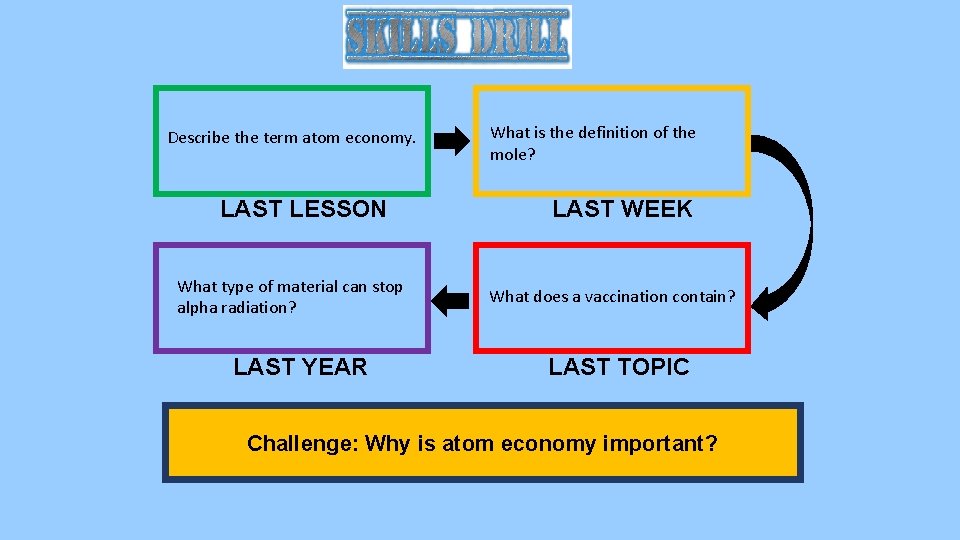
Describe the term atom economy. LAST LESSON What type of material can stop alpha radiation? LAST YEAR What is the definition of the mole? LAST WEEK What does a vaccination contain? LAST TOPIC Challenge: Why is atom economy important?

Define the word concentration Explain how the concentration of a solution in mol/dm 3 is related to the mass of the solute and the volume of the solution Calculate the concentration of one portion of a titrated solution when given the concentration of the other
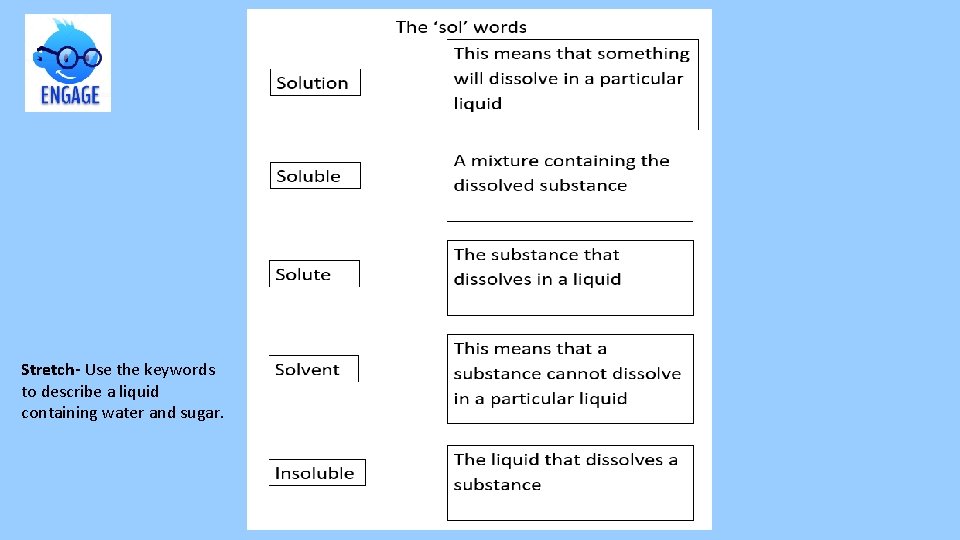
Stretch- Use the keywords to describe a liquid containing water and sugar.

Concentration You have normally come across the word concentration when dealing with cordial/squash drinks. The more water you add the less concentrated it is, or more dilute

When making a drink of orange squash, sometimes you put too much squash in the glass – sometimes you add too much water. How could you rectify both of these scenarios? A chemist would say that you are adjusting the concentration of the solution. To record, interpret and communicate their results, they need to express the concentration of the solutions they use. Chemists quote the amount of substance (solute) dissolved in a certain volume of the solution. 1. From the above sentence, what units could be used to express concentrations? 2. How do you think the concentration of a solution could be calculated?

The concentration of a solution can be changed: Concentration can be increased by dissolving more solute in a given volume of solution - this increases the mass of the solute. Concentration can be increased by allowing some of the solvent to evaporate - this decreases the volume of the solution
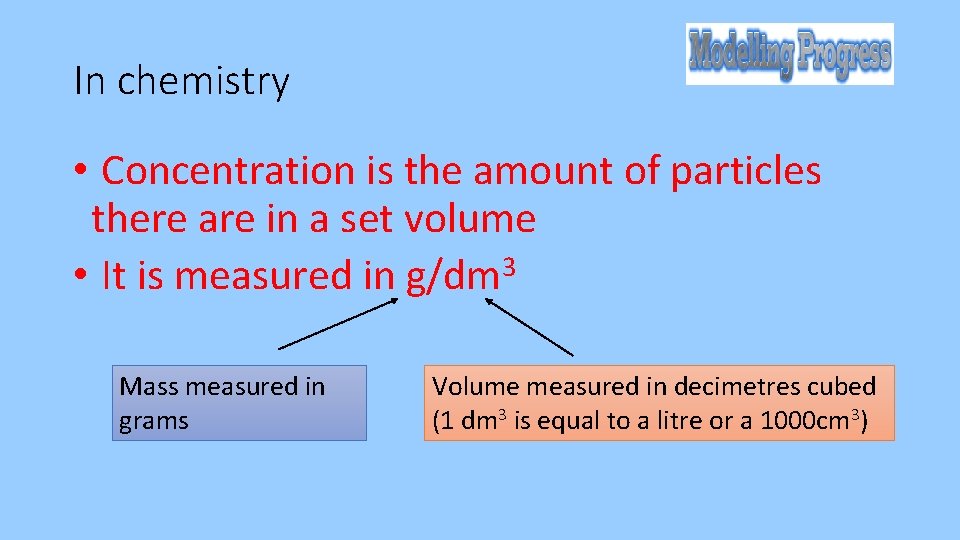
In chemistry • Concentration is the amount of particles there are in a set volume • It is measured in g/dm 3 Mass measured in grams Volume measured in decimetres cubed (1 dm 3 is equal to a litre or a 1000 cm 3)

Calculating Concentration TOGETHER: 1. What is the concentration of calcium ions in a sample of water that has: a) 0. 4 g of calcium ions dissolved in 2 dm 3 of water? b) 0. 25 g of calcium ions dissolved in 5 dm 3 of the water? Stretch: A 500 cm 3 bottle of mineral water contains 0. 2 g of magnesium ions dissolved in the water. What is the concentration of magnesium ions in g/dm 3.

Concentration (g/dm 3) = Mass (g) / Volume (dm 3) Practise: 1. You have 15 g of sugar dissolved in 0. 25 dm 3 of water – what is the concentration in g/dm 3? 2. You have 1. 5 g of sugar dissolved in 0. 25 dm 3 of water – what is the concentration in g/dm 3? 3. You have 18 g of sugar dissolved in 0. 55 dm 3 of water – what is the concentration in g/dm 3? 4. You have 150 g of sugar dissolved in 0. 75 cm 3 of water – what is the concentration in g/dm 3? 5. You have 1400 g of sugar dissolved in 25 cm 3 of water – what is the concentration in g/dm 3?

Exam Questions


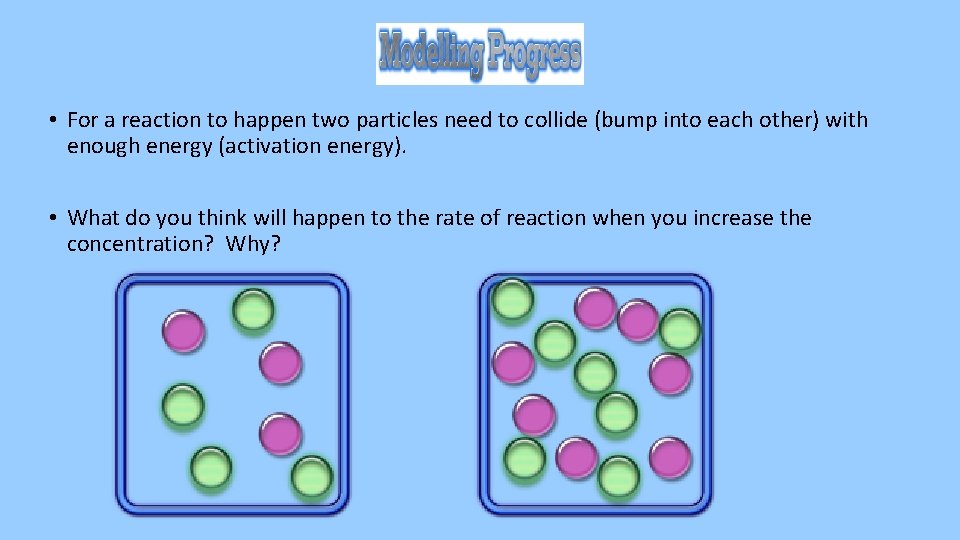
• For a reaction to happen two particles need to collide (bump into each other) with enough energy (activation energy). • What do you think will happen to the rate of reaction when you increase the concentration? Why?
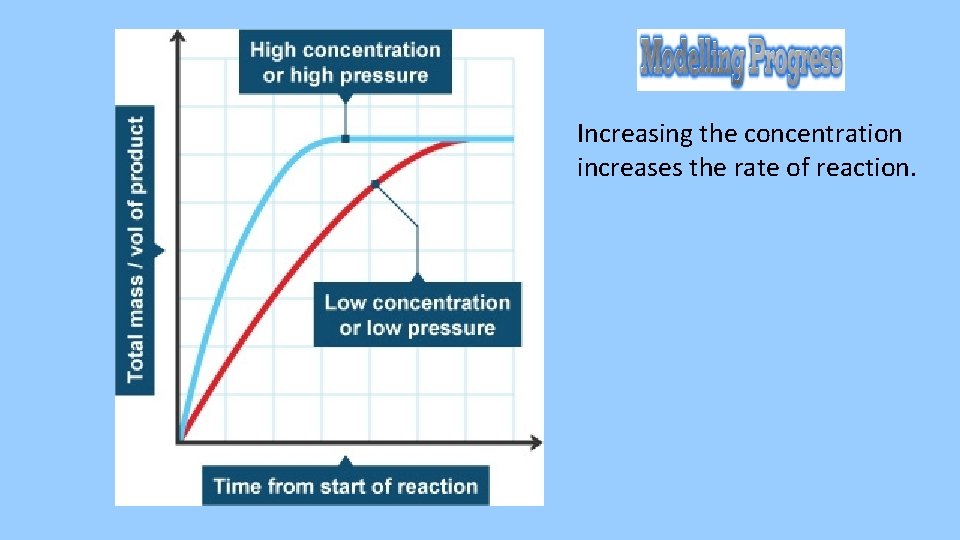
Increasing the concentration increases the rate of reaction.

Complete the following questions about our learning Todays lesson 1. Define the term concentration using sol words. 2. What unit is concentration measured in? Last lesson 3. How do you calculate atom economy? 4. Why are efficient reactions important? Last lesson 5. State 2 reasons why reactions do not always produce theoretical yield.
- Slides: 16