User Engagement Toolkit User Engagement Toolkit 1 2













































































- Slides: 80

User Engagement Toolkit

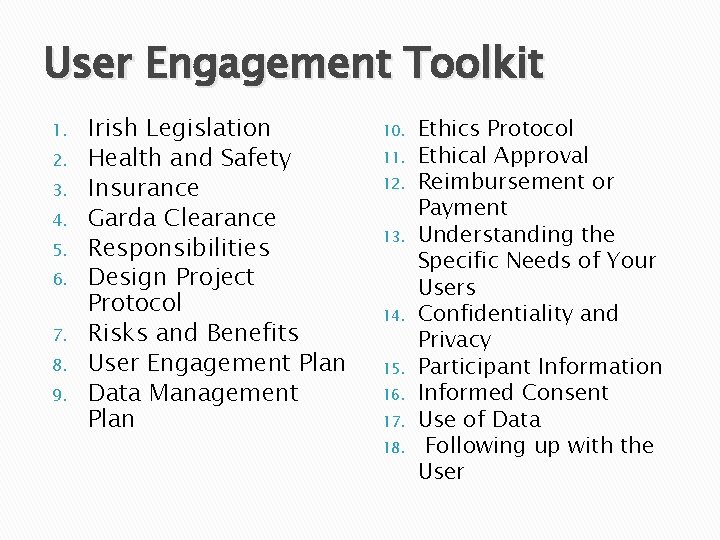
User Engagement Toolkit 1. 2. 3. 4. 5. 6. 7. 8. 9. Irish Legislation Health and Safety Insurance Garda Clearance Responsibilities Design Project Protocol Risks and Benefits User Engagement Plan Data Management Plan 10. 11. 12. 13. 14. 15. 16. 17. 18. Ethics Protocol Ethical Approval Reimbursement or Payment Understanding the Specific Needs of Your Users Confidentiality and Privacy Participant Information Informed Consent Use of Data Following up with the User

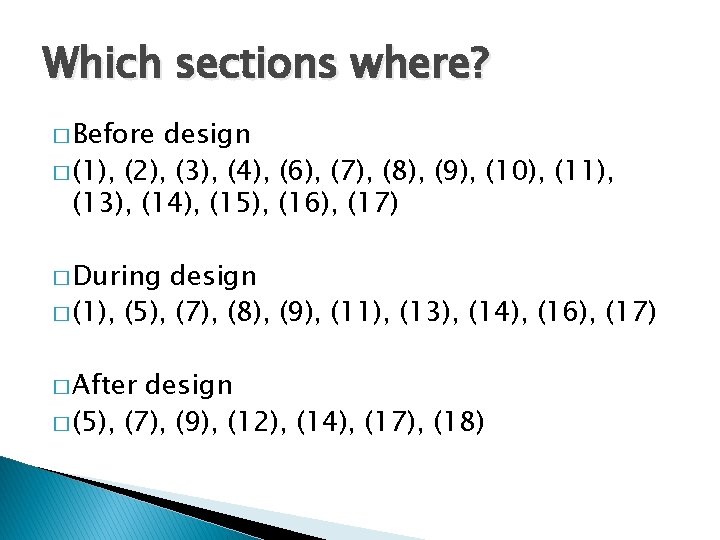
Which sections where? � Before design � (1), (2), (3), (4), (6), (7), (8), (9), (10), (11), (13), (14), (15), (16), (17) � During design � (1), (5), (7), (8), (9), (11), (13), (14), (16), (17) � After design � (5), (7), (9), (12), (14), (17), (18)

Group 1 � Barry Sheridan � Andrea Gasser � Vusala Gurbanova � Gareth Hunter � Martin Cronin

Group 2 � Joan Murphy � Peter Kovar � Danielle Pouvaneeswaree � Sameer Handa � Areej Algathani

Group 3 � Ronan Clancy � William Jones � Maryam Almussalam � Sarfraz Ramay � David Hurley

Group 4 � Atul Swami � Divyaa Manimaran � Sohaib Khan � Fei Wang � Peter Keogh

Group 5 � Alex Hannon-Cross � Dajana Davidovic � Mansour Alnakhli � Stephen Mckeown

Irish Legislation

Who � Principal Investigator ◦ Oversees/Directs all legislative responsibilities � Lecturer ◦ Understand legislation at a detailed level � Student ◦ Understand the limits imposed by legislation � User ◦ Brief understanding of legislation � Institution ◦ Defines codes of practice for user from legislation

Details � Applying Irish Legislation varies from project to project � Projects/Individuals associated with projects should identify the legal requirements in each situation � Different types of institutions have different requirements under legislation � Examples ◦ ◦ ◦ Ethics for Social Workers bye-law National Vetting Bureau European Communities Data Protection Act Freedom of Information

References � � � � � Code of Professional Conduct and Ethics for Social Workers bye-law 2011 (S. I. No. 143/2011). http: //www. irishstatutebook. ie/2011/en/si/0143. html. Accessed 18 th July 2013. The National Vetting Bureau (Children and Vulnerable Persons) Act 2012 (SI Number 47 of 2012). http: //www. irishstatutebook. ie/pdf/2012/en. act. 2012. 0047. pdf. Accessed 18 th July 2013. European Communities (Clinical Trials on Medicinal Products for Human Use) Regulations, 2004) (S. I. 190 of 2004) which implement the EU Clinical Trials Directive (2001/20/EC). http: //www. dohc. ie/other_health_issues/clinical_trials/. Accessed 18 th July 2013. Equality Authority (2011) Guide to the Equal Status Acts 2000 to 2008. http: //www. equality. ie/Files/Guide-to-the-Equal-Status-Acts-2000 -2008. pdf. Accessed 18 th July 2013. Data Protection Act 1998 (SI Number 25 of 1988) and Data Protection (Amendment) Act 2003 (SI Number 6 of 2003). http: //www. dataprotection. ie/View. Doc. asp? fn=%2 Fdocuments%2 Flegal%2 FLaw. On. DP. htm&Cat. ID =7&m=. Accessed 18 th July 2013. Data Protection Commissioner (2013). The Data Protection Rules. http: //www. dataprotection. ie/View. Doc. asp? fn=/documents/responsibilities/3 bii. htm&Cat. ID=5 4&m=y. Accessed 18 th July 2013. Citizens Information (2013). Freedom of Information in Ireland. Citizens Information website section. http: //www. citizensinformation. ie/en/government_in_ireland/national_government/standards_ and_accountability/freedom_of_information. html. Accessed 22 nd July 2013. Information on the Health Information Bill found on the Department of Health and Children website. http: //www. dohc. ie/issues/hib//. Accessed 17 th July 2013. Department of An Taoiseach website. Information on timing of the Health Information Bill. http: //www. taoiseach. gov. ie/eng/Taoiseach_and_Government/Government_Legislation_Progra mme/SECTION_B 1. html. Accessed 17 th July 2013.

Insurance

Who? � Principal Investigator ◦ Discuss user engagement activities with the relevant Institution department � Lecturer ◦ Discuss user engagement activities with the relevant Institution department � Student ◦ Ask if the appropriate insurance is in place � User ◦ Discuss concerns with the Principal Investigator, design researchers or students. � Institution ◦ Advise staff and students on insurance issues

Details ◦ Before user engagement begins, any necessary insurance arrangements need to be in place ◦ In proportion to risk ◦ Potential cover for researcher and sponsor ◦ Insurance issues going to be different according to institution ◦ Examples of potential issues

References � Irish Council for Bioethics (2004) Operational Procedures for Research Ethics Committees. � HSE (2008). Review of Research Ethics Committees and Processes in the Republic of Ireland. Research Ethics Committees Review Group 2008, Health Services Executive, Ireland. � UK Department of Health (2005). Research Governance Framework for Health and Social Care. � WHO (2011). Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants. World Health Organisation.

Garda Clearance

Who? � Principal Investigator ◦ The design project protocol should identify if vetting of staff or students is required. � Lecturer ◦ The design project protocol should identify if vetting of staff or students is required. � Student ◦ Ask if you need to get Garda clearance in advance of user engagement commencing. � User ◦ Discuss Garda vetting as an option in advance of the engagement commencing. � Institution ◦ Advise staff and students on this issue and whether or not vetting is required.

Garda Clearance If Research/Developers or Stakeholders on a project require engaging with vulnerable user groups. Adults with learning disabilities , dementia or Children. May need to be vetted by the National Vetting Bereau. Before engaging. Can take up to 12 weeks or longer.

References � Citizens Information (2013). Garda Clearance for Employees. Available at http: //www. citizensinformation. ie/en/employ ment/employment_rights_and_conditions/mo nitoring_and_surveillance_at_work/garda_clea rance_for_employees. html. Accessed 23 rd August 2013.

Health & Safety

Who � Principal Investigator ◦ Identify H&S issues and engage with appropriate departments & student � Lecturer ◦ As above � Student ◦ Follow codes of practice � User ◦ Inform Principal Investigator, researchers or students of any issues � Institution ◦ Provide guidance and training

Details � Health & Safety – to be considered from POV of DIT, researcher & user � E. g. are physical tasks are part of the study? � H&S issues with venue to be considered � Are there H&S issues in the interaction of people involved? � Seek advice! � Review Health & Safety Authority guidelines

References � Health and Safety Authority (2013). Guidelines: Your Industry. Available at http: //www. hsa. ie/eng/Your_Industry/. Accessed 23 rd August 2013.

Design Project Protocol

Who ? � Principal Investigator ◦ Prepare design project � Lecturer ◦ Prepare design project � Student ◦ Follow design project protocol � User ◦ Request a more detailed or clearer explaination � Institution ◦ Provide practical guidance

Details � Design project protocol will cover a list of topics before, during and after the project � Include a list of aims and objectives � PI create a design Protocol Template as a standard for all projects � User engagement areas should cover ◦ ◦ ◦ Risks and benefits to the user User Engagement plan Data Management plan Ethics plan User payment Follow-up with user

References � � � Durham University (2012) Community-based Participatory Research: A Guide to Ethical Principles and Practice. Centre for Social Justice and Community Action, Durham University. Irish Council for Bioethics (2004) Operational Procedures for Research Ethics Committees. National Institutes of Health (2003). Research Ethics: How to Treat People Who Participate in Research. National Institutes of Health, USA. Available at http: //www. bioethics. nih. gov/education/FNIH_Bioethics. Brochure _WEB. PDF. Accessed 1 st August 2013. NCBI (Draft) Research Code of Practice Policy and Procedure: Principles of Best Practice in Quality Research. NCBI – Working for People with Sight Loss, 2013. WHO (2011). Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants. World Health Organisation. Available at http: //whqlibdoc. who. int/publications/2011/9789241502948_e ng. pdf. Accessed 1 st August 2013.

Responsibilities

Who ? � Principal Investigator ◦ Identify roles and responsibilities. Understand relevant codes of practice, legislation & guidelines � Lecturer ◦ Identify roles and responsibilities. Understand relevant codes of practice, legislation & guidelines � Student ◦ Adhere to codes of practice and guidelines. Uphold standards when engaging with users � User ◦ Be aware of point of contact for user engagement � Institution ◦ Ensure roles and responsibilities are clearly identified. Develop code of good practice. Ensure all staff members aware of codes od practice and responsibilities.

Details � Ensure that the project is carried out to highest possible standards � Any codes or guidelines of good practice are adhered to � Clear project plan � Include the principal investigator, design student and project leader � Identify the department and staff members � Ensure all issues are addressed

References � European Science Foundation (2000). Good Scientific Practice in Research and Scholarship. European Science Foundation Policy Briefing. Available at http: //www. esf. org/fileadmin/Public_documents /Publications/ESPB 10. pdf. Accessed 1 st August 2013. � National Institutes of Health (2007). Guidelines for the Conduct of Research in the Intramural Research Programme at the National Institute of Health, USA. Available at http: //sourcebook. od. nih. gov/ethicconduct/conduct%20 research%206 -11 -07. pdf. Accessed 1 st August 2013.

User Engagement Plan

Who ? � Principal Investigator ◦ Prepare the user engagement plan � Lecturer � Prepare the user engagement plan � Student ◦ Understand user engagement plan, and prepare plans where required � User ◦ Request more detailed/clearer explanations � Institution ◦ Provide advice on how to prepare a design or protocol – including the user engagement plan

Details � Ask ◦ ◦ ◦ ◦ the following questions Who is being engaged What type of engagement is planned When will the engagement take place during the project Where will the engagement take place Why has the particular user group been selected How do you intend to use the collected data How many users do intend recruit and why ? � User engagement plan is a living document � Timeline for key actions should be included (communications, actions) � Users should be asked for their feedback

References � � � British Psychological Society Code of Conduct: Ethical Principles for Conducting Research with Human Participants. Available at http: //www. bps. org. uk/sites/default/files/documents/code_of_h uman_research_ethics. pdf. Accessed on 23 rd August 2013. Dalton, AJ, Mc. Villy, KR (2004) Ethics Guidelines for International Multi-Centre Research Involving People with Disabilities. European Commission (2013). Ethical Review in FP 7: European Commission Guidance for Applicants – Getting Through Ethical Review. Available at http: //cordis. europa. eu/fp 7/ethics_en. html. Accessed on 23 rd August 2013. RCA (2013). Design with People – Ethics Guidelines. Royal College of Art Helen Hamlyn Centre, UK. Available at http: //designingwithpeople. rca. ac. uk/ethics. Accessed on 23 rd August 2013. Sociological Association of Ireland (1997) Ethical Guidelines.

Risks & Benefits

Who ? � Principal Investigator ◦ Identify risks and benefits in advance. And advise students on how to identify them. � Lecturer � Identify risks and benefits in advance. And advise students on how to identify them. � Student ◦ Familiarise self with risks and benefits � User ◦ Ask for clarification where necessary � Institution ◦ Provide advice on how to prepare project protocols. Identify realistic risks and benefits.

Details � � Design research should have a positive effect on the lives or activities of all involved It is unethical to put people at risk of harm or discomfort Use to decide on project direction – include in participant information and informed consent Identify potential risks and benefits of the project (direct or indirect) ◦ ◦ � Physical Social Financial Psychological Identified risks ◦ should be used to Inform protocols in case of adverse events occurring ◦ Should be honest, realistic and shouldn’t coerce the user in any way � � Longevity of benefits estimation Manage user expectations

References � � � � COPE Foundation (2005) A Policy Document for Conducting Research. Durham University (2012) Community-based Participatory Research: A Guide to Ethical Principles and Practice. Centre for Social Justice and Community Action, Durham University. European Science Foundation (2000). Good Scientific Practice in Research and Scholarship. European Science Foundation Policy Briefing. Available at http: //www. esf. org/fileadmin/Public_documents/Publications/ESPB 10. pdf. Accessed 1 st August 2013. Irish Council for Bioethics (2004) Operational Procedures for Research Ethics Committees. National Disability Authority (2009) Ethical Guidance for Research with People with Disabilities. National Disability Authority, Ireland. National Institutes of Health (2003). Research Ethics: How to Treat People Who Participate in Research. National Institutes of Health, USA. Available at http: //www. bioethics. nih. gov/education/FNIH_Bioethics. Brochure_WEB. PDF. Accessed 1 st August 2013. Sociological Association of Ireland (1997) Ethical Guidelines. WHO (2011). Standards and Operational Guidance for Ethics Review of Health. Related Research with Human Participants. World Health Organisation. Available at http: //whqlibdoc. who. int/publications/2011/9789241502948_eng. pdf. Accessed 1 st August 2013.

Data Management Plan

Who? � Principal Investigator ◦ Create a data managment plan and advise the student to create their own plan. � Lecturer ◦ Same as above � Student ◦ Follow data management plan identified by principal investigator � User ◦ Ask for more details or more explanation. If the information provided to you isn’t clear � Institution ◦ Provide advise to prepare design or research project protocol.

Data Management Plan -Details � Many designer may be they are unaware of the importance of maintaining a high standard regard to user data management. � All users should be treated the same level of confidentially and privacy � Learning a good practice in data management will prepare you for managing complex sets of user data in the future. � At least question why you are creating a data management plan. � A data management plan should cover every single piece of personal and other data collected about user. � Be aware of Irish Data Protection rules.

References � � � Durham University (2012) Community-based Participatory Research: A Guide to Ethical Principles and Practice. Centre for Social Justice and Community Action, Durham University. European Science Foundation (2000). Good Scientific Practice in Research and Scholarship. European Science Foundation Policy Briefing. Available at http: //www. esf. org/fileadmin/Public_documents/Publications/ESPB 10. pdf. Accessed 1 st August 2013. UK Medical Research Council (2012) Good Research Practice: Principles and Guidelines. UK Medical Research Council (2013). MRC Guidance on Data Management Plans. UK Medical Research Council Website. Available at http: //www. mrc. ac. uk/Ourresearch/Ethicsresearchguidance/datasharing/DMPs/in dex. htm. Accessed 23 rd August 2013. National Institutes of Health (2007). Guidelines for the Conduct of Research in the Intramural Research Programme at the National Institute of Health, USA. Available at http: //sourcebook. od. nih. gov/ethic-conduct/conduct%20 research%206 -1107. pdf. Accessed 1 st August 2013. NCBI (Draft) Research Code of Practice Policy and Procedure: Principles of Best Practice in Quality Research. NCBI – Working for People with Sight Loss, 2013.

Ethics Protocol

Who? � Principal Investigator � Lecturer � Student � User � Institution ◦ Prepares ethics protocol and advises students how to prepare their own. ◦ Same as above ◦ Follows ethics protocol identified by principal investigator or where required prepares protocol. ◦ Participant information should explain ethical issues otherwise a clear explanation should be asked for. ◦ Prepares a code of practice for design research ethics and raises awareness of ethical issues that should be addressed in design projects. Provides guidance on how to prepare ethics protocol and obtain informed consent.

Ethics Protocol - Details � Should be used when planning a design project that includes user engagement � Outlines potential ethical issues and the steps to address the issues � Acts as a record of unexpected ethics issues � Contain a user engagement plan, preparing data management plan and getting informed consent � Contain information on how informed consent is obtained, recruiting in an ethical manner treatment of users, treatment of personal information etc.

References British Psychological Society Code of Conduct: Ethical Principles for Conducting Research with Human Participants. Available at http: //www. bps. org. uk/sites/default/files/documents/cod e_of_human_research_ethics. pdf. Accessed on 23 rd August 2013. � Dalton, AJ, Mc. Villy, KR (2004) Ethics Guidelines for International Multi-Centre Research Involving People with Disabilities. � European Commission (2013). Ethical Review in FP 7: European Commission Guidance for Applicants – Getting Through Ethical Review. Available at http: //cordis. europa. eu/fp 7/ethics_en. html. Accessed on 23 rd August 2013. � Sociological Association of Ireland (1997) Ethical Guidelines. �

Ethical Approval

Who? � Principal Investigator ◦ Finding research ethics committee and apply for approval and identify any ethics requirements � Lecturer ◦ Same as above � Student ◦ Follow the principal investigators approval instructions � User ◦ If unclear ethical issues are provided to ask for more details � Institution ◦ Set up a research ethics committee or find one and to set up codes of practice and training if needed.

Ethical Approval - Details � Check what rules or requirements are for the school/institution or department � Check if a research ethics committee exists if not identify one and seek its approval � Check if you need approval from external organisation or board. � Check if source of funding requires ethical approval

References � � � � HSE (2008). Review of Research Ethics Committees and Processes in the Republic of Ireland. Research Ethics Committees Review Group 2008, Health Services Executive, Ireland. Irish Council for Bioethics (2004) Operational Procedures for Research Ethics Committees. Irish Medicines Board (2013). Medical Devices. Available at http: //www. imb. ie/EN/Medical-Devices. aspx. Accessed on 23 rd August 2013. NDA (2009). Ethical Guidance for Research with People with Disabilities. Disability Research Series 13. National Disability Authority, Ireland. Privireal (2005). Ireland - RECs and Medical Research. Available at http: //www. privireal. org/content/rec/ireland. php. Accessed on 23 rd August 2013. Sociological Association of Ireland (1997) Ethical Guidelines. WHO (2011). Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants. World Health Organisation.

Reimbursement of Payment

Who? � Principal Investigator ◦ Consider the cost of user engagement and method for reimbursement. � Lecturer ◦ Consider personal details that will be required from the users for payment. � Student ◦ Collect necessary information from users so that payment process is smooth. � User ◦ Document the receipt of payment. Make it easy for principal investigator by providing all the necessary relevant info. � Institution ◦ Improve coordination between finance department and principal investigator to make process efficient.

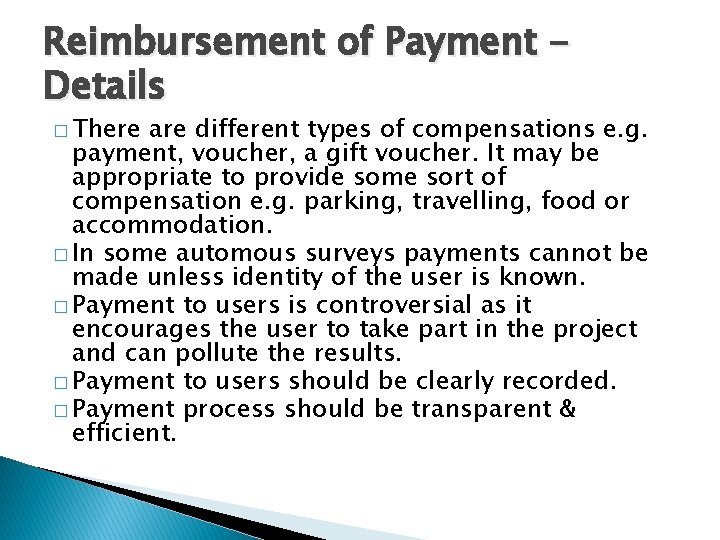
Reimbursement of Payment Details � There are different types of compensations e. g. payment, voucher, a gift voucher. It may be appropriate to provide some sort of compensation e. g. parking, travelling, food or accommodation. � In some automous surveys payments cannot be made unless identity of the user is known. � Payment to users is controversial as it encourages the user to take part in the project and can pollute the results. � Payment to users should be clearly recorded. � Payment process should be transparent & efficient.

References � National Disability Authority (2009) Ethical Guidance for Research with People with Disabilities. National Disability Authority, Ireland. � National Institutes of Health (2007). Guidelines for the Conduct of Research in the Intramural Research Programme at the National Institute of Health, USA. Available at http: //sourcebook. od. nih. gov/ethicconduct/conduct%20 research%206 -11 -07. pdf. Accessed 1 st August 2013. � WHO (2011). Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants. World Health Organisation. Available at http: //whqlibdoc. who. int/publications/2011/978924 1502948_eng. pdf. Accessed 1 st August 2013.

Understanding the Specific Needs of Your Users

Who? � Principal Investigator ◦ Responsible for identifying user needs and to address them. � Lecturer ◦ Same as Principal Investigator � Student ◦ Educate yourself on extreme user needs and follow investigator’s guidance. � User ◦ Providing particular requirements to the researchers. � Institution ◦ To educate the staff about the needs of older and disabled people and how to collect information in an accessible format.

Details � � � Most of the times requirements are almost same to every user but in some cases, requirements may be specific. The best approach to find out if someone has specific requirements is to get engage with them and simply ask. To ensure that user engagement goes smoothly, they should be provided comfort and easy access to the venue and activities. And it is important to provide users the complete and clear details of the user engagement program. And while compiling any thing that serves the purpose of the communication, we should follow some protocols so that our words be heard and read by all users, regardless of their age, or disability. And its wise to know your users earliest as possible.

References � ISO (2001) ISO/IEC Guide 71: 2001. Guidelines for Standards Developers to Address the Needs of Older Persons and Persons with Disabilities. � NDA (2013). Centre for Excellence in Universal Design website (Customer Engagement Toolkits). Available at www. universaldesign. ie. Accessed 23 rd August 2013. � NDA (2002). Guidelines for Including People with Disabilities in Research. National Disability Authority, Ireland. � NDA (2002). Ask Me: Guidelines for Effective Consultation with People with Disabilities. National Disability Authority, Ireland.

Confidentiality and Privacy

Who? � Principal Investigator ◦ Familiar with user data best practice & keeping students informed of those practices. � Lecturer ◦ Same as Principal Investigator � Student ◦ Follow guidance of PI and learn methods of data sensitivity � User ◦ Understand rights and why the data is being collected. Don’t provide data you don’t feel comfortable providing � Institution ◦ Develop protocols and educate staff on data collection issues.

Details � Always treat data with confidentiality and privacy. � Educate anyone with access to the data about their responsibilities � Store data securely � Separate data from users identification � Transport data securely � People should not be identifiable without permission � When sharing data, balance data protection while getting the most out of the data. Don’t protect data to the point where datasets need to be recreated unnecessarily

References � � � Data Protection Commissioner (2013). The Data Protection Rules. http: //www. dataprotection. ie/View. Doc. asp? fn=/documents/resp onsibilities/3 bii. htm&Cat. ID=54&m=y. Accessed 18 th July 2013. Durham University (2012) Community-based Participatory Research: A Guide to Ethical Principles and Practice. Centre for Social Justice and Community Action, Durham University. European Science Foundation (2000). Good Scientific Practice in Research and Scholarship. European Science Foundation Policy Briefing. Available at http: //www. esf. org/fileadmin/Public_documents/Publications/ES PB 10. pdf. Accessed 1 st August 2013. Medical Research Council (2012) Good Research Practice: Principles and Guidelines. NCBI (Draft) Research Code of Practice Policy and Procedure: Principles of Best Practice in Quality Research. NCBI – Working for People with Sight Loss, 2013. Sociological Association of Ireland (1997) Ethical Guidelines.

Participant Information 65

Who Does What? � Principle Investigator – Prepare clear concise Participant Information. Advise students on preparation of Participant Information. � Lecturer- Prepare clear concise Participant Information. � Student- Preparation and communication of Participant Information. � User – Request appropriate information format; if necessary ask for more details � Institute – Guidelines/advice to preparation of Participant Information. 66

Details - - - Crucial: Clear and understandable information (in recruitment stage or introduction to Informed Consent) Format of the Participant Information must comply with user access, so that specific user needs are met (wrt written or f 2 f communication) Include User Understanding and Freedom, see p. 3 Include project details, its purpose, the procedure and contact details Give one copy of PI to the user; keep one signed version 67

References � Code of Professional Conduct and Ethics for Social Workers bye-law 2011 (S. I. No. 143/2011). http: //www. irishstatutebook. ie/2011/en/si/014 3. html. Accessed 18 th July 2013. � Irish Council for Bioethics (2004) Operational Procedures for Research Ethics Committees. � NDA (2009). Ethical Guidance for Research with People with Disabilities. Disability Research Series 13. National Disability Authority, Ireland. � WHO (2011). Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants. World Health Organisation.

Informed Consent 69

Who Does What? � Principle Investigator – Co-ordinator � Lecturer- Advisor � Student- Collector � User – Inform � Institute – Guidelines and codes 70

Details Start of the engagement process � User Understanding and Freedom ◦ Implications of the project ◦ Freedom of participation ◦ Ability to withdraw � Be careful of anonymity, use of photo’s and videos � Acquire proxy consent � Proxy consent and assent � Factors influencing users ◦ ◦ Knowing the person Developing a relationship Professional connection Skewing information through reimbursement or payment 71

References � Dalton, AJ, Mc. Villy, KR (2004) Ethics Guidelines for International Multi-Centre Research Involving People with Disabilities. � Irish Council for Bioethics (2004) Operational Procedures for Research Ethics Committees. � NDA (2009). Ethical Guidance for Research with People with Disabilities. Disability Research Series 13. National Disability Authority, Ireland. � National Institutes of Health (2003). Research Ethics: How to Treat People Who Participate in Research. National Institutes of Health, USA. Available at http: //www. bioethics. nih. gov/education/FNIH_Bioethi cs. Brochure_WEB. PDF. Accessed 1 st August 2013.

Use of Data 73

Who Does What? � Principle Investigator – User data � Lecturer- User Data � Student- Follow Guidance , IP � User – If short information, more details � Institute – Education & Guidance 74

Details � Stick to the agreement of the data use � In case of change, inform the user � Confidentiality & privacy � Maybe IP agreement 75

References � � � Durham University (2012) Community-based Participatory Research: A Guide to Ethical Principles and Practice. Centre for Social Justice and Community Action, Durham University. European Science Foundation (2000). Good Scientific Practice in Research and Scholarship. European Science Foundation Policy Briefing. Available at http: //www. esf. org/fileadmin/Public_documents/Publications/ES PB 10. pdf. Accessed 1 st August 2013. Health Research Board (2007) Health Research Board Guidelines for Host Institutions on Good Research Practice. Medical Research Council (2012) Good Research Practice: Principles and Guidelines. NDA (2009). Ethical Guidance for Research with People with Disabilities. Disability Research Series 13. National Disability Authority, Ireland. Sociological Association of Ireland (1997) Ethical Guidelines.

Following up on the User 77

Who Does What? � Principle Investigator – Co-ordinator/Advisor � Lecturer-Co-ordinator/Advisor � Student- Follow Guidance , Actioner � User – Inquire about design project aspects � Institute – Advice on Policy 78

Details � Send Thank you � Possible reimbursement or payment � Hear final findings � Form of recognition or acknowledgement � Peer review stage user included � Format accessible to user. 79

References � Durham University (2012) Community-based Participatory Research: A Guide to Ethical Principles and Practice. Centre for Social Justice and Community Action, Durham University. � Irish Council for Bioethics (2010) Recommendations for Promoting Research Integrity. � National Institutes of Health (2003). Research Ethics: How to Treat People Who Participate in Research. National Institutes of Health, USA. Available at http: //www. bioethics. nih. gov/education/FNIH_Bioethi cs. Brochure_WEB. PDF. Accessed 1 st August 2013. � NDA (2002). Ask Me: Guidelines for Effective Consultation with People with Disabilities. National Disability Authority, Ireland.