Use of Solar energy Conversion of solar energy


- Slides: 12


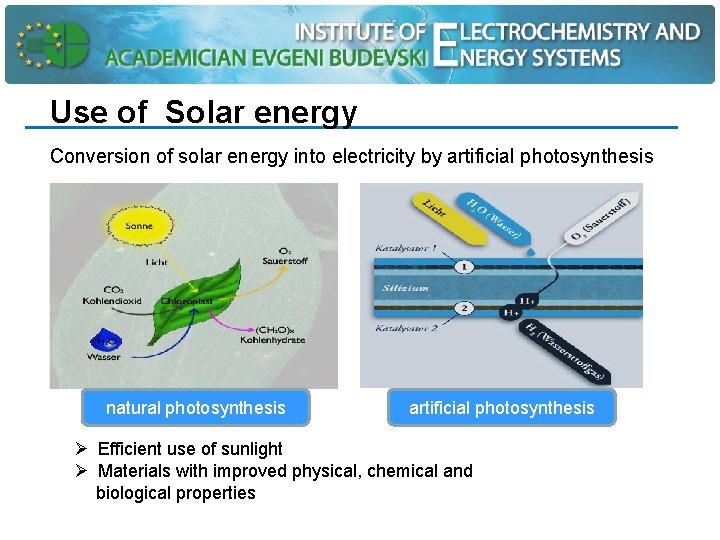
Use of Solar energy Conversion of solar energy into electricity by artificial photosynthesis natural photosynthesis artificial photosynthesis Ø Efficient use of sunlight Ø Materials with improved physical, chemical and biological properties

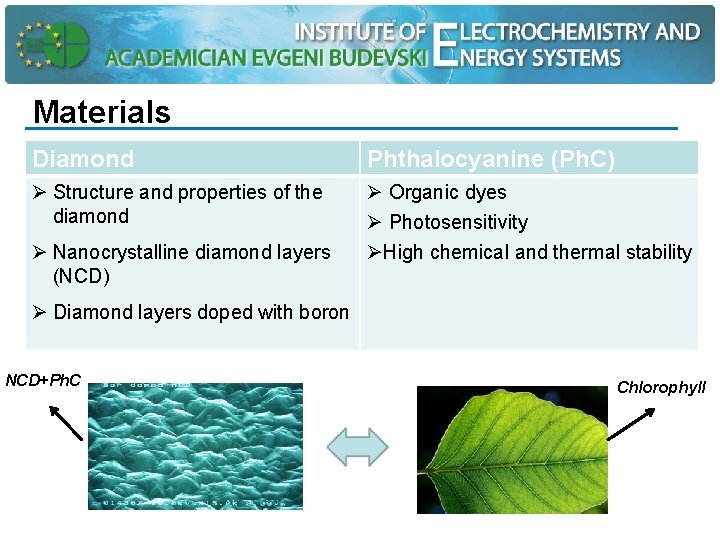
Materials Diamond Phthalocyanine (Ph. C) Ø Structure and properties of the diamond Ø Organic dyes Ø Photosensitivity ØHigh chemical and thermal stability Ø Nanocrystalline diamond layers (NCD) Ø Diamond layers doped with boron NCD+Ph. C Chlorophyll

Sequence of the experiment 1. Deposition of nanocrystalline layers 2. Modification of diamond surfaces - O 2 -plasma - NH 3 -plasma - UV/O 3 -treatment 3. Immobilization with photosensitive molecules 4. Analyzing the effectiveness of the modification - Measurement of contact angles - X-ray photoelectron spectroscopy - Raman spectroscopy 5. Fotoelektrochemical research

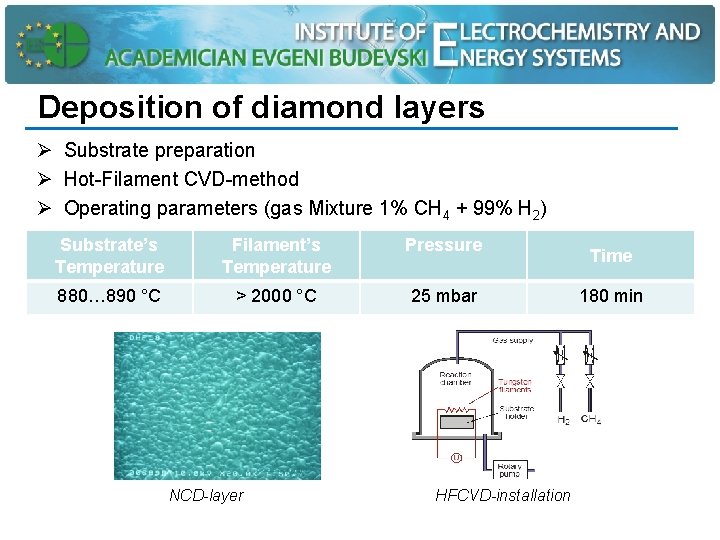
Deposition of diamond layers Ø Substrate preparation Ø Hot-Filament CVD-method Ø Operating parameters (gas Mixture 1% CH 4 + 99% H 2) Substrate’s Temperature Filament’s Temperature Pressure 880… 890 °C > 2000 °C 25 mbar NCD-layer HFCVD-installation Time 180 min

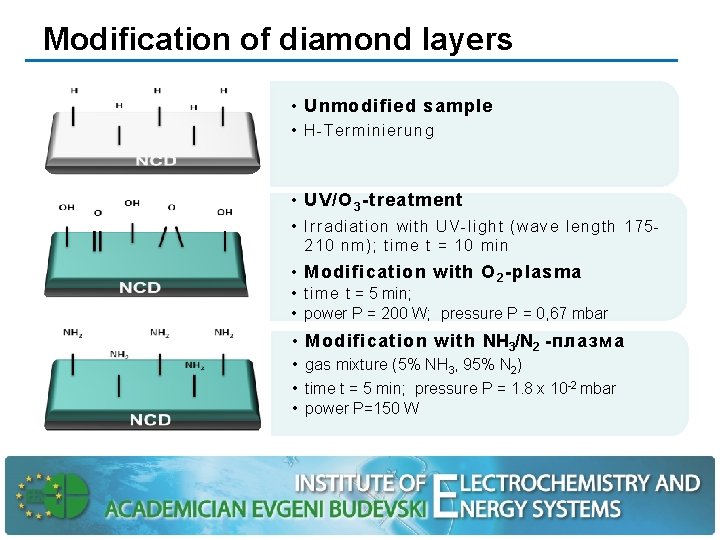
Modification of diamond layers • Unmodified sample • H-Terminierung • UV/O 3 -treatment • Irradiation with UV-light (wave length 175210 nm); time t = 10 min • Modification with O 2 -plasma • time t = 5 min; • power P = 200 W; pressure P = 0, 67 mbar • Modification with NH 3/N 2 -плазма • gas mixture (5% NH 3, 95% N 2) • time t = 5 min; pressure P = 1. 8 x 10 -2 mbar • power P=150 W

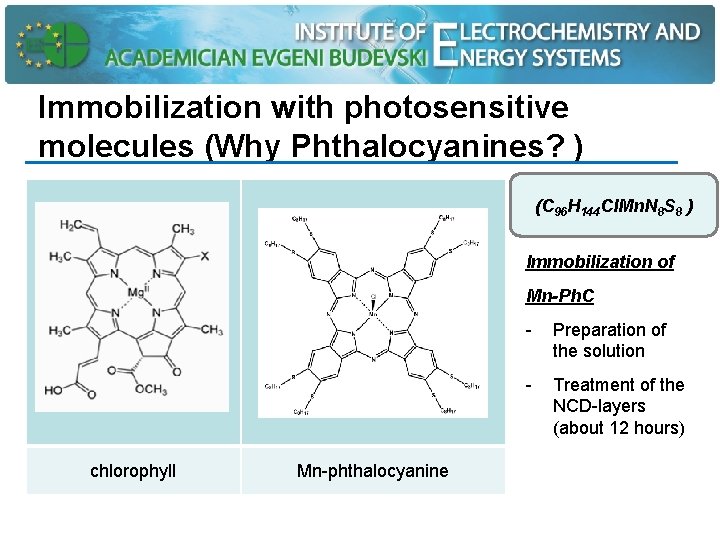
Immobilization with photosensitive molecules (Why Phthalocyanines? ) (C 96 H 144 Cl. Mn. N 8 S 8 ) Immobilization of Mn-Ph. C chlorophyll Mn-phthalocyanine - Preparation of the solution - Treatment of the NCD-layers (about 12 hours)

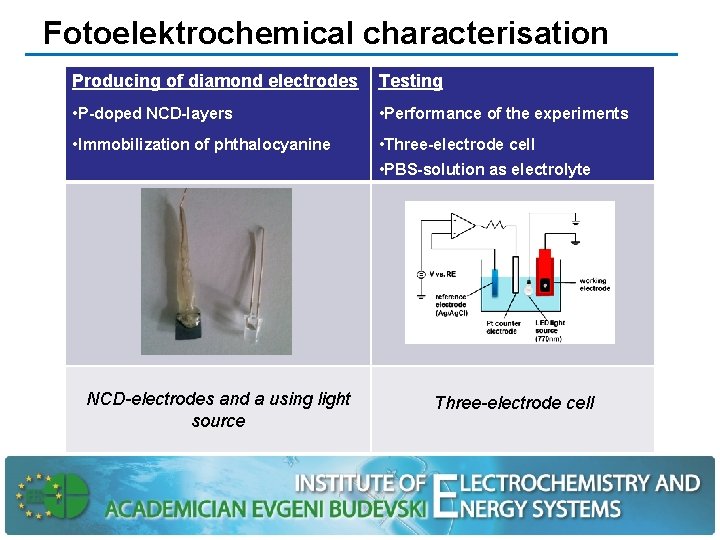
Fotoelektrochemical characterisation Producing of diamond electrodes Testing • P-doped NCD-layers • Performance of the experiments • Immobilization of phthalocyanine • Three-electrode cell • PBS-solution as electrolyte NCD-electrodes and a using light source Three-electrode cell

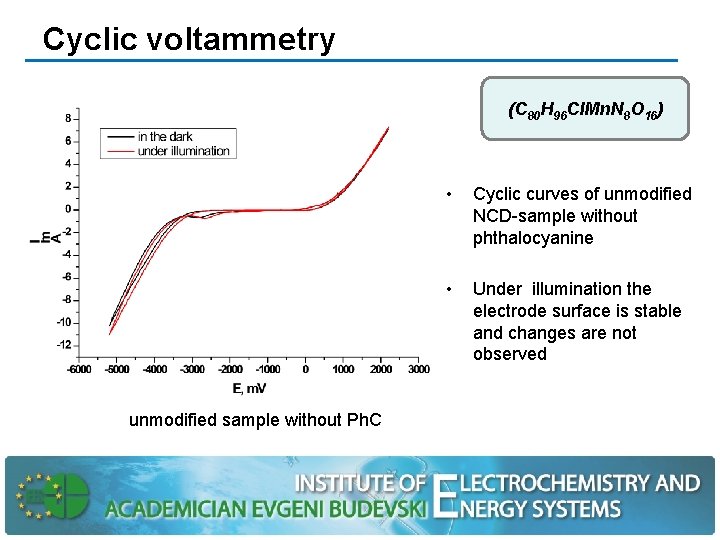
Cyclic voltammetry (C 80 H 96 Cl. Mn. N 8 О 16) unmodified sample without Ph. C • Cyclic curves of unmodified NCD-sample without phthalocyanine • Under illumination the electrode surface is stable and changes are not observed

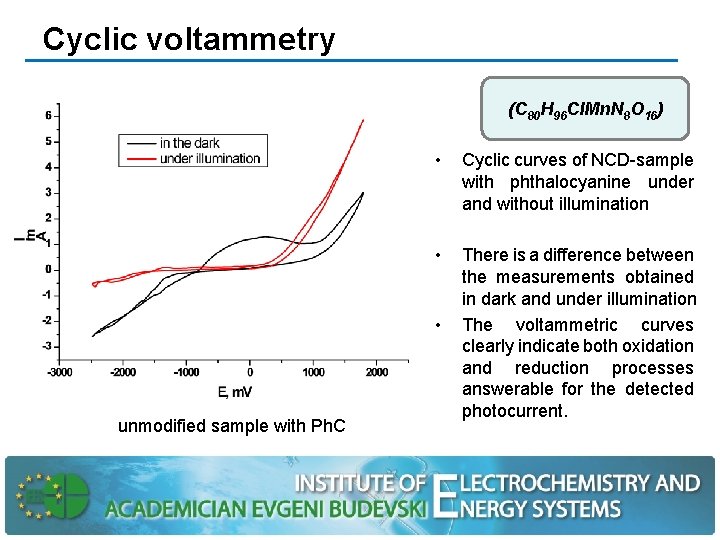
Cyclic voltammetry (C 80 H 96 Cl. Mn. N 8 О 16) • Cyclic curves of NCD-sample with phthalocyanine under and without illumination • There is a difference between the measurements obtained in dark and under illumination The voltammetric curves clearly indicate both oxidation and reduction processes answerable for the detected photocurrent. • unmodified sample with Ph. C

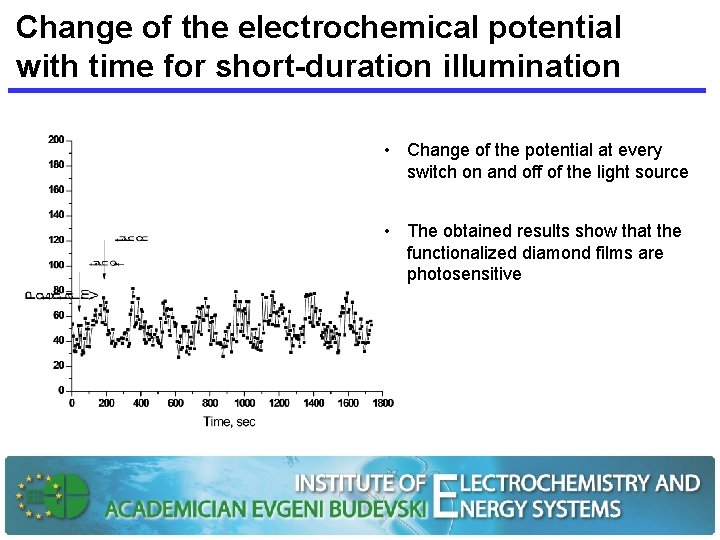
Change of the electrochemical potential with time for short-duration illumination • Change of the potential at every switch on and off of the light source • The obtained results show that the functionalized diamond films are photosensitive

 Solar energy is free. solar is inexhaustible
Solar energy is free. solar is inexhaustible Energy conversion of a toaster
Energy conversion of a toaster Indirect solar energy
Indirect solar energy Ladder conversion
Ladder conversion Write the correct abbreviation for each metric unit answers
Write the correct abbreviation for each metric unit answers Metric stair case
Metric stair case Wholesale panel solar cell
Wholesale panel solar cell What is wind energy conversion system
What is wind energy conversion system Energy transformation in electric kettle
Energy transformation in electric kettle Energy transformation in a blender
Energy transformation in a blender Energy transformation of an oven
Energy transformation of an oven Energy conversion and conservation
Energy conversion and conservation Fossil fuels examples
Fossil fuels examples