Use of Pharmacokinetics Modeling Simulation in the Drug













- Slides: 22

Use of Pharmacokinetics Modeling & Simulation in the Drug Development: From Discovery to Development Joseph Kim, Ph. D, RPh Director Clinical Pharmacology Modeling & Simulation GSK, R&D, Research Triangle Park

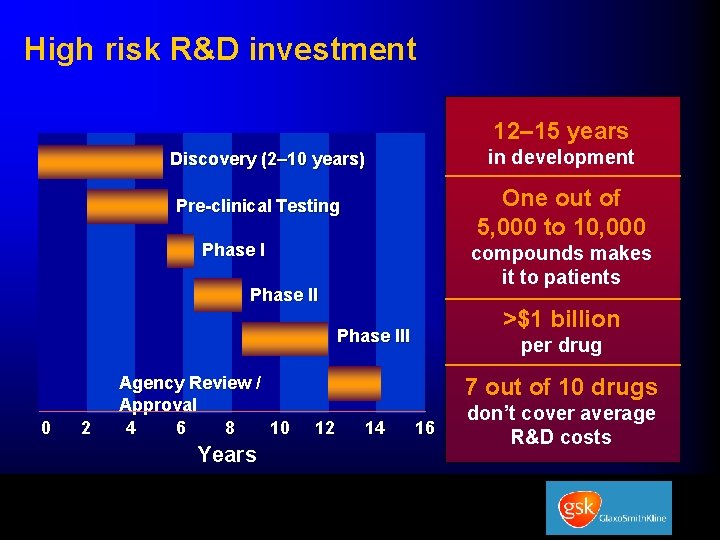
High risk R&D investment 12– 15 years in development Discovery (2– 10 years) One out of 5, 000 to 10, 000 Pre-clinical Testing Phase I compounds makes it to patients Phase II >$1 billion Phase III 0 2 Agency Review / Approval 4 6 8 10 Years per drug 7 out of 10 drugs 12 14 16 don’t cover average R&D costs

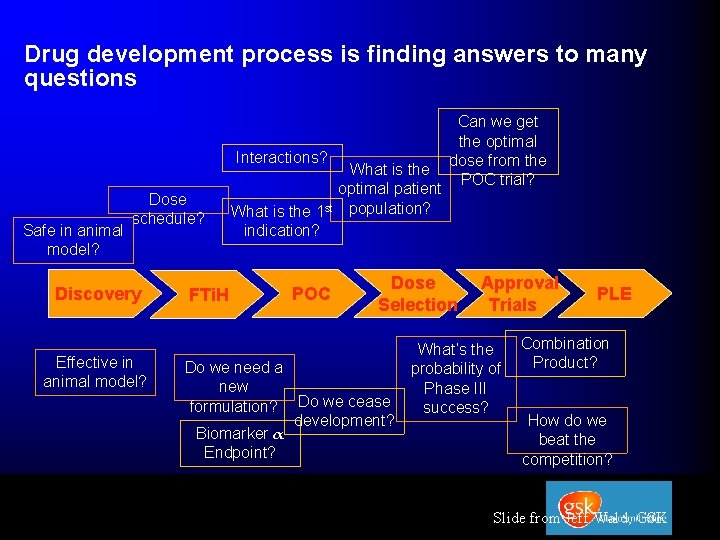
Drug development process is finding answers to many questions Interactions? Safe in animal model? Dose schedule? Discovery Effective in animal model? FTi. H What is the optimal patient What is the 1 st population? indication? POC Can we get the optimal dose from the POC trial? Dose Selection Do we need a new formulation? Do we cease development? Biomarker α Endpoint? Approval Trials What’s the probability of Phase III success? PLE Combination Product? How do we beat the competition? Slide from Jeff Wald, GSK

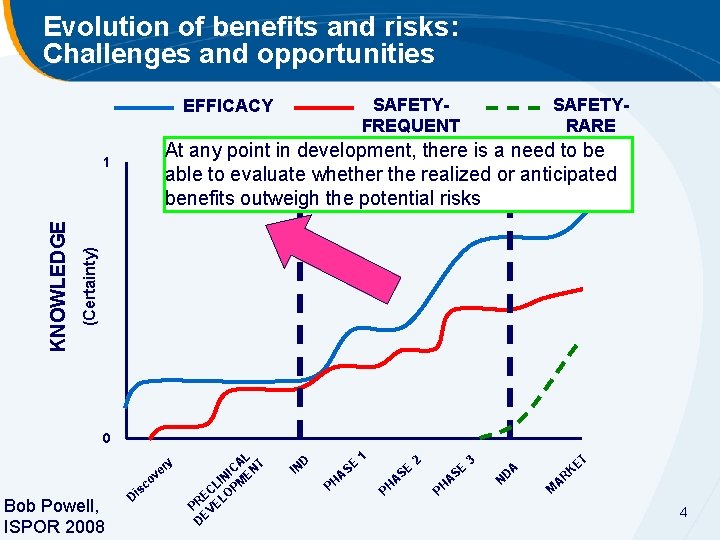
Evolution of benefits and risks: Challenges and opportunities SAFETYFREQUENT EFFICACY At any point in development, there is a need to be able to evaluate whether the realized or anticipated benefits outweigh the potential risks (Certainty) 1 KNOWLEDGE SAFETYRARE 0 ry ve co Bob Powell, ISPOR 2008 s Di L CA NT I IN ME L P EC LO R P VE DE D IN PH E AS 1 PH E AS 2 PH E AS 3 T N DA E RK A M 4

Clinical Pharmacology: Study of drug in humans* – Pharmacokinetics – Pharmacodynamics – Drug Metabolism and Transport – Optimizing and Evaluating Patient Therapy – Drug Discovery and Development *Principles of Clinical Pharmacology, 2 nd Ed. Arthur Adkison et al.

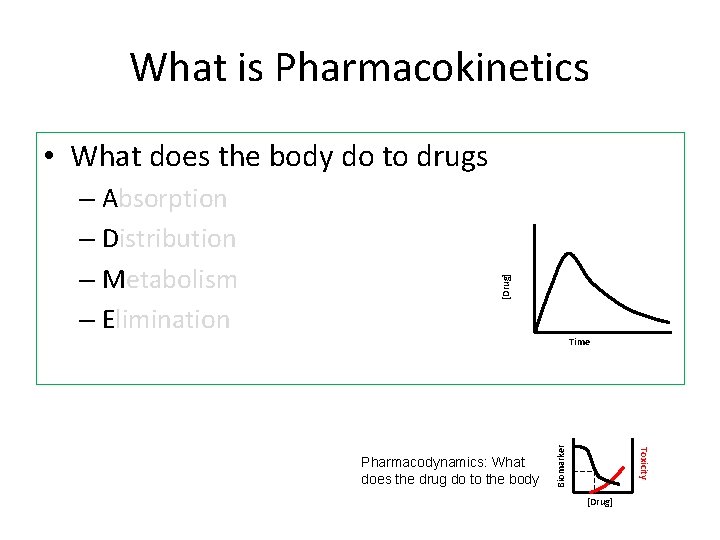
What is Pharmacokinetics Time Toxicity Pharmacodynamics: What does the drug do to the body Biomarker – Absorption – Distribution – Metabolism – Elimination [Drug] • What does the body do to drugs [Drug]

PK Use in Drug Development Discovery (pre-clinical) Phase I – First Time in Human study (FTi. H) Phase I – Non- FTi. H study Phase II – Proof of Concept, Proof of Mechanism, Dose Finding study • Phase III – Pivotal studies • Phase IV – Post approval commitment, PLE(Product Line Extension), Country specific registrations • •

PK Use in Drug Development • Discovery (pre-clinical) – Allometric scaling> Modeling & Simulation – Predict AUC, Cmax, target dose – Suggest dose strength – Compare human exposure to animal toxicity (NOAEL- No Observed Adverse Effect Level)

Case #1: Use of Modeling & Simulation with Animal toxicokinetic study · Single dose rat study – no toxicity but some unusual TK profile · Repeat dose rat study initiated – animal dies with 1500 mg/kg dose after 3 -4 days · Single dose study TK profile reviewed and reevaluated using modeling & simulation

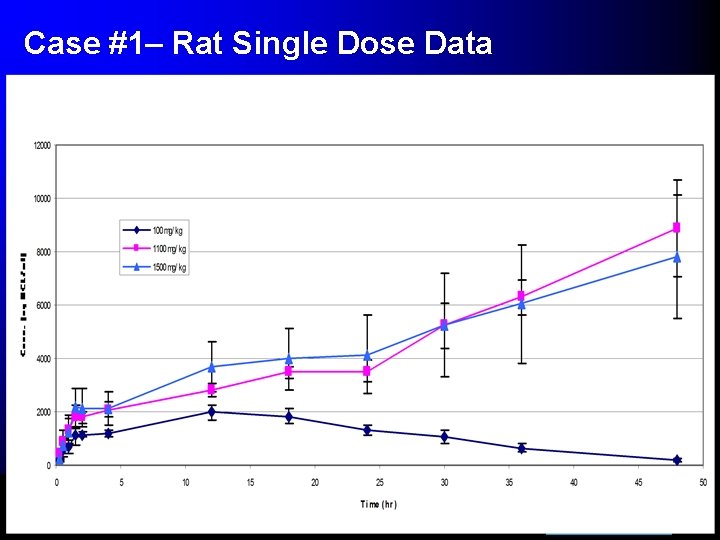
Case #1– Rat Single Dose Data

Snapshot of 300 mg/kg Dose for SD and Repeat Dose • Significant accumulation, 4 fold increase in AUClast • Elimination phase has not begun at 24 hrs. on neither day 1 or day 7

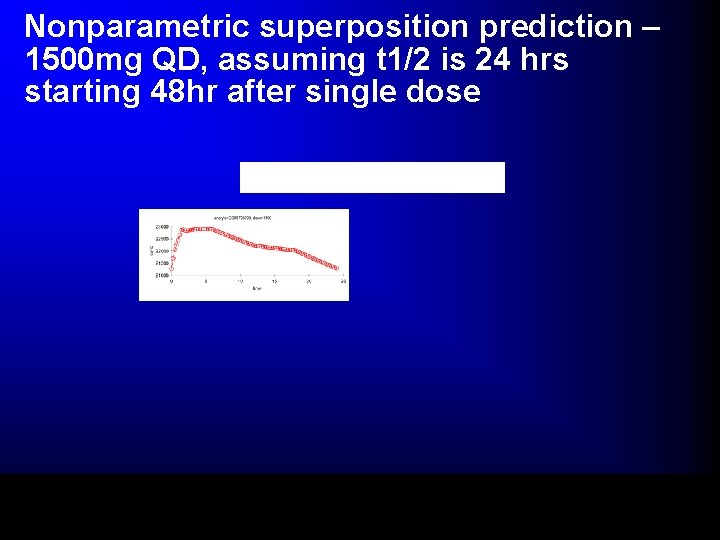
Nonparametric superposition prediction – 1500 mg QD, assuming t 1/2 is 24 hrs starting 48 hr after single dose

PK Use in Drug Development • Phase I – First Time in Human study (FTi. H) – Single Dose Study>> Dose escalation – Repeat Dose Study>> Dose escalation – PK data analysis and Modeling & Simulation – Predict AUC, Cmax, target dose – Compare human exposure to animal toxicity (NOAEL- No Observed Adverse Effect Level) or biological activity (MABEL – Minimum Anticipated Biological Effect Level)

PK Use in Drug Development • Phase I – After FTi. H – – – – Formulation study Food effect study Drug Interaction study (perpetrator vs victim) Renal impairment study Hepatic impairment study QTc study Others (pediatric, elderly population) Compare human exposure to animal toxicity (NOAEL- No Observed Adverse Effect Level)

PK Use in Drug Development • Phase II – Proof of Concept, Proof of Mechanism, Dose Finding study – Modeling & Simulation – Predict target site concentration and needed dose – Use protein binding, receptor occupancy etc. – Suggest dose and regimen for Phase III – Prepare Eo. P 2 a meeting with regulatory agency – Predict probability of success with given dose and variability – Compare human exposure to animal toxicity (NOAEL- No Observed Adverse Effect Level)

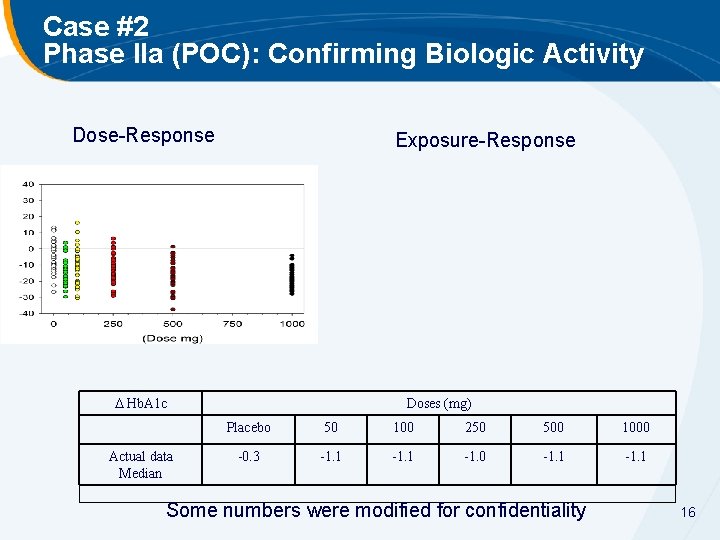
Case #2 Phase IIa (POC): Confirming Biologic Activity Dose-Response Exposure-Response Hb. A 1 c Actual data Median Doses (mg) Placebo 50 100 250 500 1000 -0. 3 -1. 1 -1. 0 -1. 1 Some numbers were modified for confidentiality 16

PK Use in Drug Development • Phase III – Pivotal studies – Population PK and Modeling & Simulation – Adaptive Design – Ethnopharmacology – Pharmacogenetics – Regulatory filings

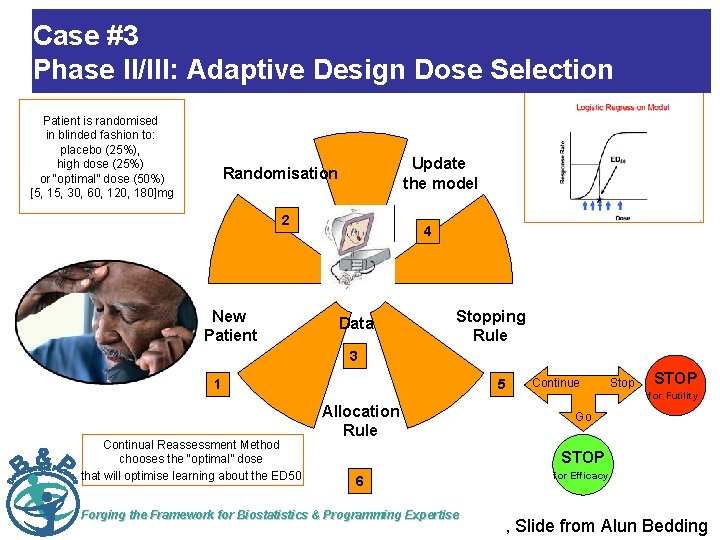
Case #3 Phase II/III: Adaptive Design Dose Selection Patient is randomised in blinded fashion to: placebo (25%), high dose (25%) or “optimal” dose (50%) [5, 15, 30, 60, 120, 180]mg Update the model Randomisation 2 New Patient 4 Data Stopping Rule 3 1 Continual Reassessment Method chooses the “optimal” dose that will optimise learning about the ED 50 5 Allocation Rule Continue Stop STOP for Futility Go STOP 6 Forging the Framework for Biostatistics & Programming Expertise for Efficacy , Slide from Alun Bedding

PK Use in Drug Development • Phase IV – Post approval commitment, PLE, and Country specific registrations – Population PK and Modeling & Simulation – Bioequivalence study – Foreign country registration – Regulatory filings

A model-based continuum provides a quantitative framework for the expected…and unexpected questions LB Sheiner, Clinical Pharmacology and Therapeutics, March 1997 Lalonde, et al. , Clinical Pharmacology and Therapeutics, July 2007 20

Summary • Drug Development is very high-risk business with big • • • uncertainty and variability Public and FDA ask more efficient way of Drug Development PK/PD modeling and simulation can answer many questions in every stage of Drug Development Model-based drug development/adaptive design approaches are powerful tools to improve clinical drug development, regulatory guidance and the quality of NDA submissions USA FDA is aware and willing to accept innovations in New Drug Development Processes Choosing the right dose is extremely important 21

Acknowledgements and References • GSK R&D Colleagues-- Jeff Wald, Patrick Ryan, Alun Bedding • Bob Powell (formerly at FDA) 22