Use of Oncotype Dx Testing Breast SSG meeting

Use of Oncotype Dx® Testing Breast SSG meeting 10 th July 2015 Dr Rebecca Bowen
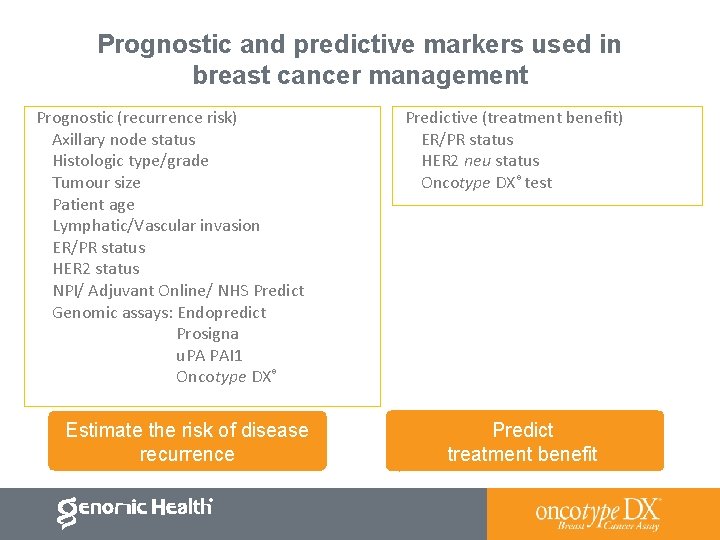
Prognostic and predictive markers used in breast cancer management Prognostic (recurrence risk) Axillary node status Histologic type/grade Tumour size Patient age Lymphatic/Vascular invasion ER/PR status HER 2 status NPI/ Adjuvant Online/ NHS Predict Genomic assays: Endopredict Prosigna u. PA PAI 1 Oncotype DX® Estimate the risk of disease recurrence Predictive (treatment benefit) ER/PR status HER 2 neu status Oncotype DX® test Predict treatment benefit
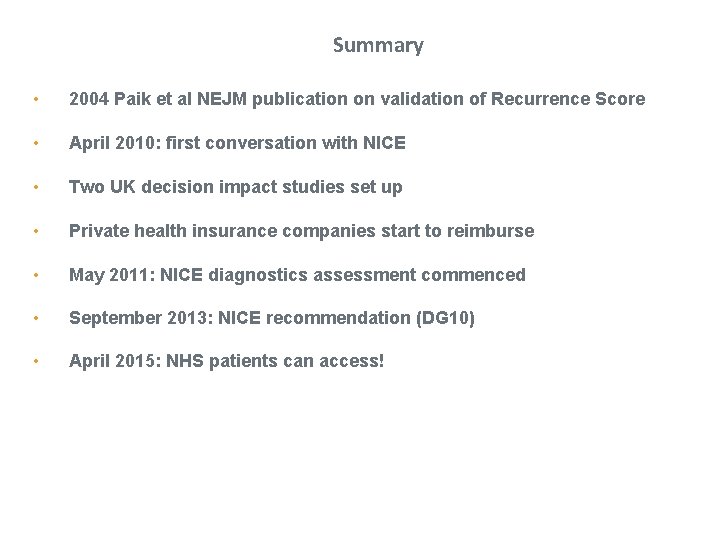
Summary • 2004 Paik et al NEJM publication on validation of Recurrence Score • April 2010: first conversation with NICE • Two UK decision impact studies set up • Private health insurance companies start to reimburse • May 2011: NICE diagnostics assessment commenced • September 2013: NICE recommendation (DG 10) • April 2015: NHS patients can access!
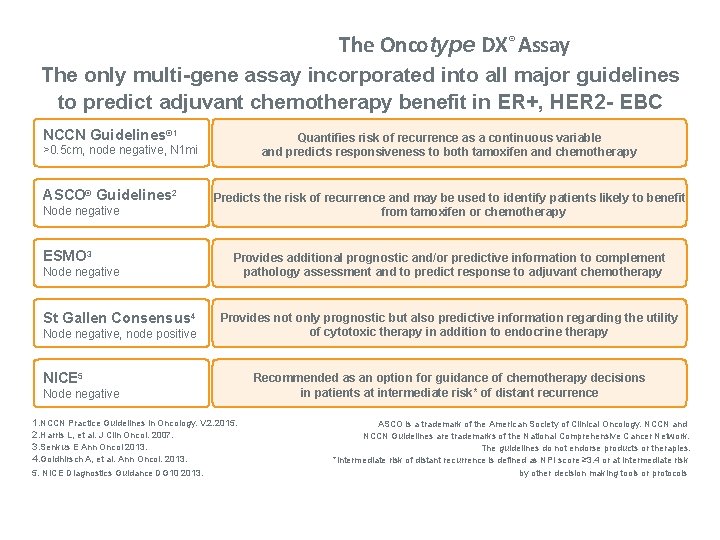
The Oncotype DX® Assay The only multi-gene assay incorporated into all major guidelines to predict adjuvant chemotherapy benefit in ER+, HER 2 - EBC NCCN Guidelines® 1 Quantifies risk of recurrence as a continuous variable and predicts responsiveness to both tamoxifen and chemotherapy >0. 5 cm, node negative, N 1 mi ASCO® Guidelines 2 Node negative ESMO 3 Node negative St Gallen Consensus 4 Node negative, node positive Predicts the risk of recurrence and may be used to identify patients likely to benefit from tamoxifen or chemotherapy Provides additional prognostic and/or predictive information to complement pathology assessment and to predict response to adjuvant chemotherapy Provides not only prognostic but also predictive information regarding the utility of cytotoxic therapy in addition to endocrine therapy NICE 5 Node negative 1. NCCN Practice Guidelines in Oncology. V. 2. 2015. 2. Harris L, et al. J Clin Oncol. 2007. 3. Senkus E Ann Oncol 2013. 4. Goldhirsch A, et al. Ann Oncol. 2013. 5. NICE Diagnostics Guidance DG 10 2013. Recommended as an option for guidance of chemotherapy decisions in patients at intermediate risk* of distant recurrence ASCO is a trademark of the American Society of Clinical Oncology. NCCN and NCCN Guidelines are trademarks of the National Comprehensive Cancer Network. The guidelines do not endorse products or therapies. *Intermediate risk of distant recurrence is defined as NPI score ≥ 3. 4 or at intermediate risk by other decision making tools or protocols
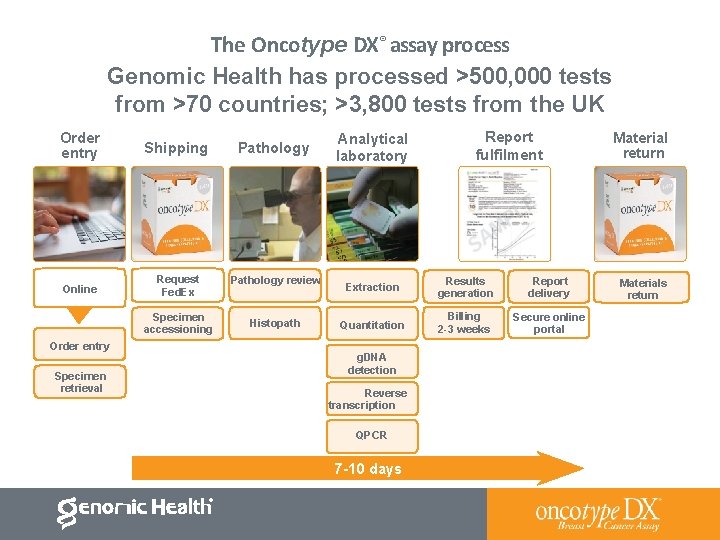
The Oncotype DX® assay process Genomic Health has processed >500, 000 tests from >70 countries; >3, 800 tests from the UK Order entry Shipping Pathology Online Request Fed. Ex Pathology review Specimen accessioning Histopath Order entry Specimen retrieval Analytical laboratory Report fulfilment Extraction Results generation Report delivery Quantitation Billing 2 -3 weeks Secure online portal g. DNA detection Reverse transcription QPCR 7 -10 days Material return Materials return
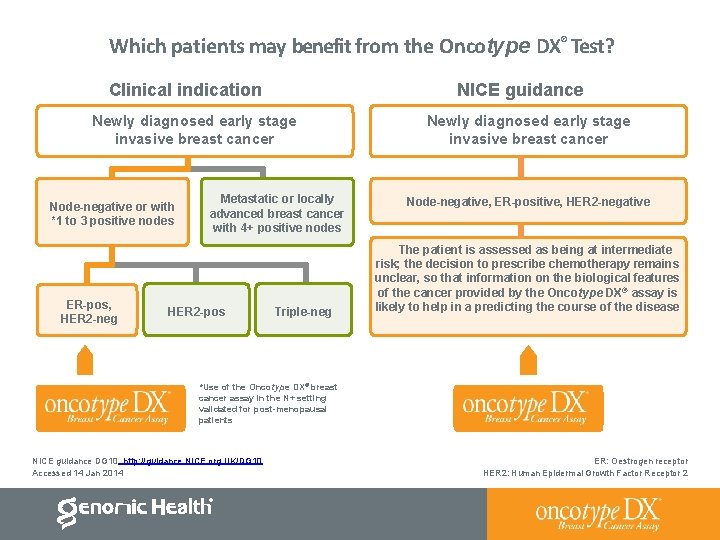
Which patients may benefit from the Oncotype DX® Test? Clinical indication NICE guidance Newly diagnosed early stage invasive breast cancer Node-negative or with *1 to 3 positive nodes ER-pos, HER 2 -neg Metastatic or locally advanced breast cancer with 4+ positive nodes HER 2 -pos Triple-neg Newly diagnosed early stage invasive breast cancer Node-negative, ER-positive, HER 2 -negative The patient is assessed as being at intermediate risk; the decision to prescribe chemotherapy remains unclear, so that information on the biological features of the cancer provided by the Oncotype DX® assay is likely to help in a predicting the course of the disease *Use of the Oncotype DX® breast cancer assay in the N+ setting validated for post-menopausal patients NICE guidance DG 10. http: //guidance. NICE. org. UK/DG 10 Accessed 14 Jan 2014 ER: Oestrogen receptor HER 2: Human Epidermal Growth Factor Receptor 2
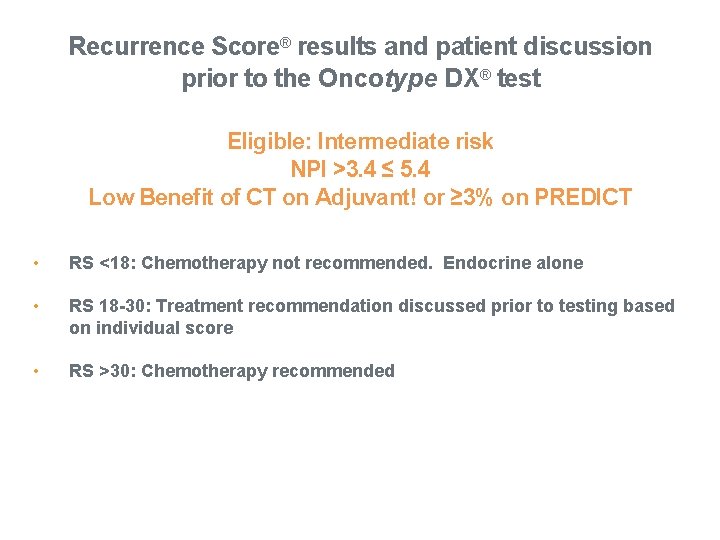
Recurrence Score® results and patient discussion prior to the Oncotype DX® test Eligible: Intermediate risk NPI >3. 4 ≤ 5. 4 Low Benefit of CT on Adjuvant! or ≥ 3% on PREDICT • RS <18: Chemotherapy not recommended. Endocrine alone • RS 18 -30: Treatment recommendation discussed prior to testing based on individual score • RS >30: Chemotherapy recommended
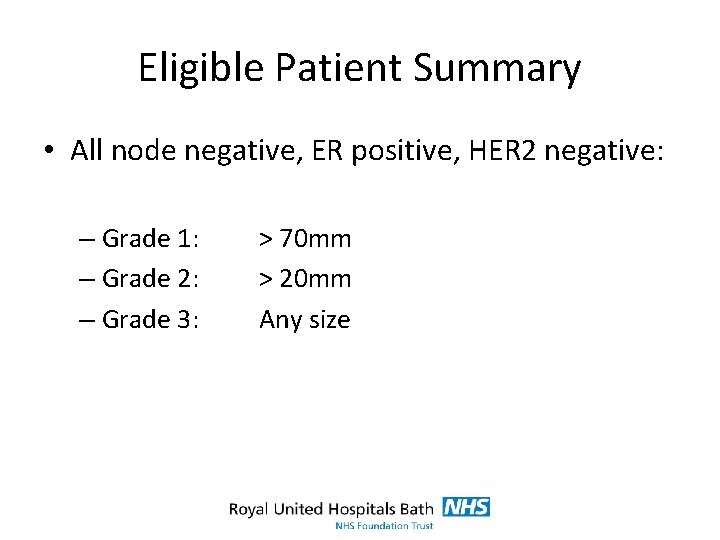
Eligible Patient Summary • All node negative, ER positive, HER 2 negative: – Grade 1: – Grade 2: – Grade 3: > 70 mm > 20 mm Any size

Change in Recommendations – Choice Study • Oncologists recommendation changed in 55 (42%) patients • 43 chemotherapy to no chemotherapy • 12 no chemotherapy to chemotherapy • Oncologists recommended chemotherapy in 51% patients pre Oncotype DX and 28% post Oncotype DX • Changes in recommendation were consistent with the Recurrence Score
- Slides: 11