Urological Tumour Group National Perspective Data from Portfolio



- Slides: 15

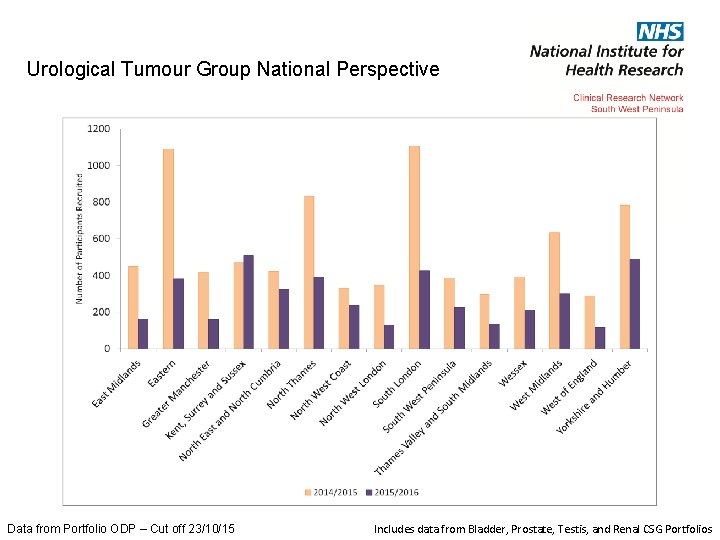
Urological Tumour Group National Perspective Data from Portfolio ODP – Cut off 23/10/15 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

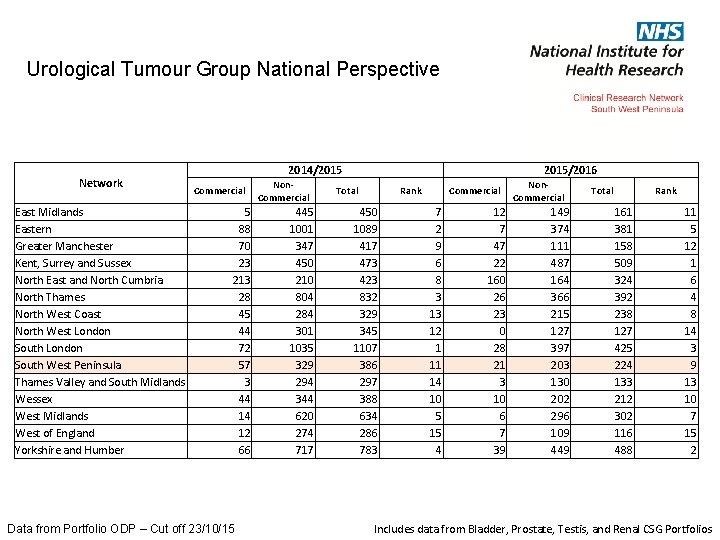
Urological Tumour Group National Perspective Network East Midlands Eastern Greater Manchester Kent, Surrey and Sussex North East and North Cumbria North Thames North West Coast North West London South West Peninsula Thames Valley and South Midlands Wessex West Midlands West of England Yorkshire and Humber 2014/2015 Commercial 5 88 70 23 213 28 45 44 72 57 3 44 14 12 66 Data from Portfolio ODP – Cut off 23/10/15 Non. Commercial 445 1001 347 450 210 804 284 301 1035 329 294 344 620 274 717 2015/2016 Total Rank 450 1089 417 473 423 832 329 345 1107 386 297 388 634 286 783 Commercial 7 2 9 6 8 3 13 12 1 11 14 10 5 15 4 12 7 47 22 160 26 23 0 28 21 3 10 6 7 39 Non. Commercial 149 374 111 487 164 366 215 127 397 203 130 202 296 109 449 Total Rank 161 381 158 509 324 392 238 127 425 224 133 212 302 116 488 11 5 12 1 6 4 8 14 3 9 13 10 7 15 2 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

CANC - 3577 - PRIME - PRostate Imaging for Margin Evaluation CANC - 3742 - REASSURE CANC - 4362 - CICERO: Cystoscopic Imaging Collection and Enhancement p. ROject NCRN - 2484 / SPARTAN - ARN-509 in non-metastatic castration-resistant prostate ca NCRN - 2835: Patterns of Care and Outcomes of Men with Prostate Cancer NCRN - 3012 Olaparib/placebo + abiraterone in m. CRPC following prior treatment with docextaxel NCRN - 3089 Radium-223 in patients with bone predominant metastatic CRPC NCRN - 3312 - MPDL 3280 A VS Chemotherapy in UROTHELIAL Bladder Cancer NCRN 428 Biop. Save - Validation of a novel proteomic blood test for prostate ca diagnosis NCRN 452 - registry of pts with metastatic castrate-resistant prostate ca NCRN 456 - DCVAC / Pca vs Placebo in Prostate Cancer NCRN 541 - Abiraterone with Different Steroid Regimens in Prostate Cancer Data from Portfolio ODP – Cut off 23/10/15 0 7 0 1 0 0 0 4 0 0 0 0 5 0 0 0 11 19 0 0 123 0 0 6 6 0 0 9 0 0 8 0 0 5 0 0 0 0 0 1 3 0 1 0 0 1 23 10 0 0 491 0 0 106 17 0 0 49 0 3 3 4 3 1 6 0 0 0 13 0 0 0 0 20 0 0 3 4 0 0 1 0 0 7 0 0 0 6 2 3 3 0 0 20 114 0 23 0 0 11 0 0 0 3 0 13 0 4 Y&H TV&SM Wes WM Wo. E SWP NT NWC NWL SL NE&NC KSS Study GM EM E Urological Tumour Group Open Commercial Studies 0 0 0 4 0 0 0 2 0 3 0 0 2 4 0 0 0 139 5 0 0 0 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

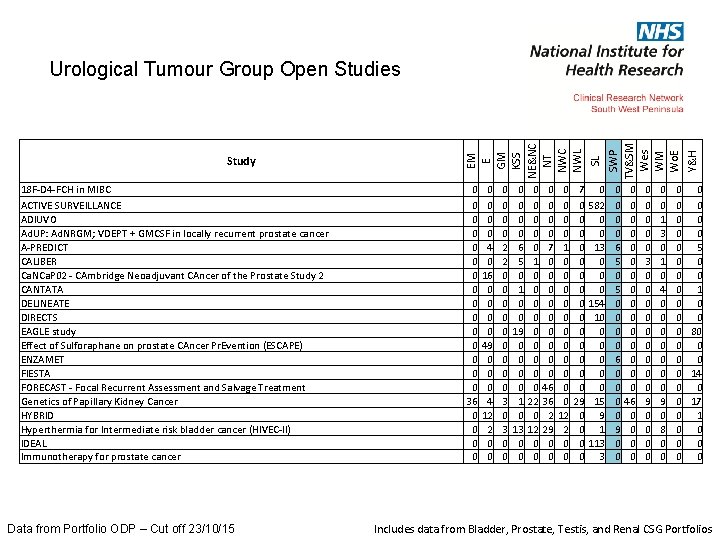
18 F-D 4 -FCH in MIBC ACTIVE SURVEILLANCE ADIUVO Ad. UP: Ad. NRGM; VDEPT + GMCSF in locally recurrent prostate cancer A-PREDICT CALIBER Ca. NCa. P 02 - CAmbridge Neoadjuvant CAncer of the Prostate Study 2 CANTATA DELINEATE DIRECTS EAGLE study Effect of Sulforaphane on prostate CAncer Pr. Evention (ESCAPE) ENZAMET FIESTA FORECAST - Focal Recurrent Assessment and Salvage Treatment Genetics of Papillary Kidney Cancer HYBRID Hyperthermia for Intermediate risk bladder cancer (HIVEC-II) IDEAL Immunotherapy for prostate cancer Data from Portfolio ODP – Cut off 23/10/15 0 582 0 0 13 0 0 0 154 10 0 0 15 9 1 113 3 0 0 4 0 16 0 0 49 0 0 0 4 12 2 0 0 0 0 0 3 0 0 0 6 5 0 1 0 0 19 0 0 1 0 13 0 0 0 0 1 0 0 0 0 0 22 0 12 0 0 0 7 0 0 0 0 0 46 36 2 29 0 0 0 1 0 0 0 12 2 0 0 7 0 0 0 0 29 0 0 0 0 6 5 0 0 0 0 6 0 0 9 0 0 0 0 0 46 0 0 0 0 0 3 0 0 0 0 0 9 0 0 0 1 3 0 1 0 4 0 0 0 0 9 0 8 0 0 Y&H 0 0 0 0 36 0 0 SWP TV&SM Wes WM Wo. E SL Study EM E GM KSS NE&NC NT NWC NWL Urological Tumour Group Open Studies 0 0 0 0 0 5 0 0 0 1 0 0 0 80 0 0 14 0 0 0 17 0 1 0 0 0 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

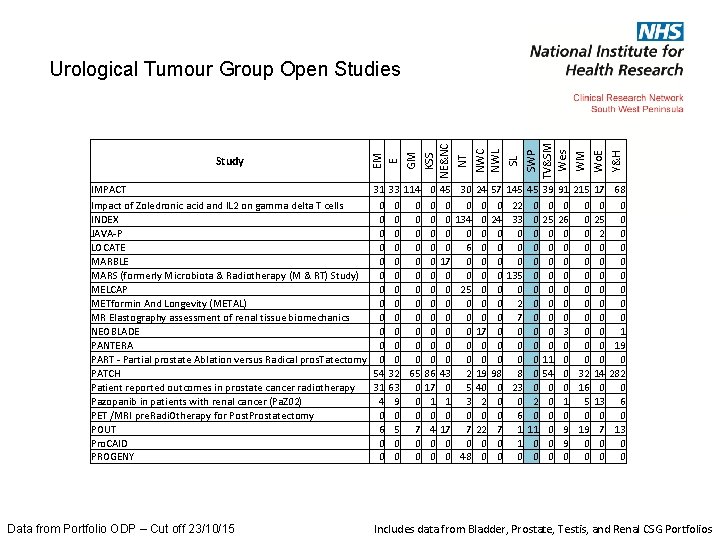
Data from Portfolio ODP – Cut off 23/10/15 SL SWP TV&SM Wes WM Wo. E Y&H IMPACT 31 33 114 0 45 30 Impact of Zoledronic acid and IL 2 on gamma delta T cells 0 0 0 INDEX 0 0 0 134 JAVA-P 0 0 0 LOCATE 0 0 0 6 MARBLE 0 0 17 0 MARS (formerly Microbiota & Radiotherapy (M & RT) Study) 0 0 0 MELCAP 0 0 0 25 METformin And Longevity (METAL) 0 0 0 MR Elastography assessment of renal tissue biomechanics 0 0 0 NEOBLADE 0 0 0 PANTERA 0 0 0 PART - Partial prostate Ablation versus Radical pros. Tatectomy 0 0 0 PATCH 54 32 65 86 43 2 Patient reported outcomes in prostate cancer radiotherapy 31 63 0 17 0 5 Pazopanib in patients with renal cancer (Pa. Z 02) 4 9 0 1 1 3 PET /MRI pre. Radi. Otherapy for Post. Prostatectomy 0 0 0 POUT 6 5 7 4 17 7 Pro. CAID 0 0 0 PROGENY 0 0 0 48 NWC NWL NT KSS NE&NC GM Study EM E Urological Tumour Group Open Studies 24 0 0 0 0 0 17 0 0 19 40 2 0 22 0 0 145 22 33 0 0 0 135 0 2 7 0 0 0 8 23 0 6 1 1 0 45 0 0 0 0 2 0 11 0 0 215 0 0 0 32 16 5 0 19 0 0 17 0 25 2 0 0 0 0 0 14 0 13 0 7 0 0 68 0 0 0 0 0 1 19 0 282 0 6 0 13 0 0 57 0 24 0 0 0 0 0 98 0 0 0 7 0 0 39 0 25 0 0 0 0 0 11 54 0 0 0 91 0 26 0 0 0 0 3 0 0 1 0 9 9 0 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

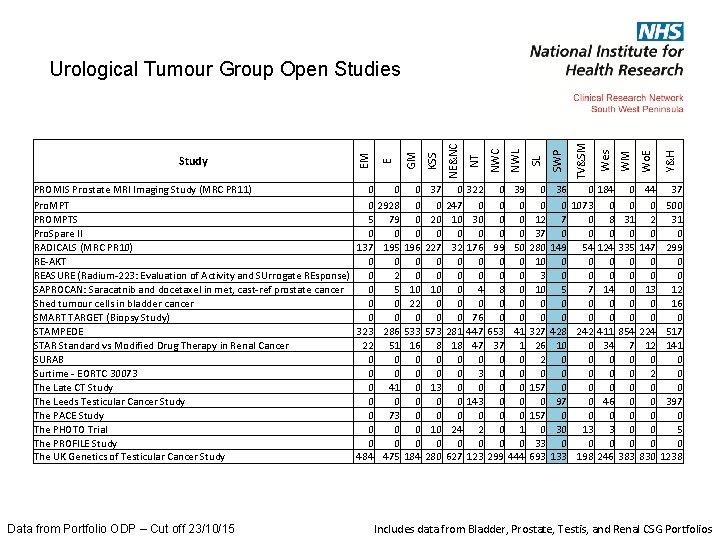
Y&H Wo. E SWP Data from Portfolio ODP – Cut off 23/10/15 WM SL 36 0 184 0 44 37 0 1073 0 0 0 500 7 0 8 31 2 31 0 0 0 149 54 124 335 147 299 0 0 0 5 7 14 0 13 12 0 0 0 16 0 0 0 428 242 411 854 224 517 10 0 34 7 12 141 0 0 0 0 0 2 0 0 0 0 97 0 46 0 0 397 0 0 0 30 13 3 0 0 5 0 0 0 133 198 246 383 830 1238 Wes NWL TV&SM NWC 0 0 12 37 280 10 3 10 0 0 327 26 2 0 157 0 33 693 KSS 39 0 0 0 50 0 0 41 1 0 0 0 1 0 444 GM 0 0 99 0 0 8 0 0 653 37 0 0 0 0 299 E PROMIS Prostate MRI Imaging Study (MRC PR 11) 0 0 0 37 0 322 Pro. MPT 0 2928 0 0 247 0 PROMPTS 5 79 0 20 10 30 Pro. Spare II 0 0 0 RADICALS (MRC PR 10) 137 195 196 227 32 176 RE-AKT 0 0 0 REASURE (Radium-223: Evaluation of Activity and SUrrogate REsponse) 0 2 0 0 SAPROCAN: Saracatnib and docetaxel in met, cast-ref prostate cancer 0 5 10 10 0 4 Shed tumour cells in bladder cancer 0 0 22 0 0 0 SMART TARGET (Biopsy Study) 0 0 0 76 STAMPEDE 323 286 533 573 281 447 STAR Standard vs Modified Drug Therapy in Renal Cancer 22 51 16 8 18 47 SURAB 0 0 0 Surtime - EORTC 30073 0 0 0 3 The Late CT Study 0 41 0 13 0 0 The Leeds Testicular Cancer Study 0 0 0 143 The PACE Study 0 73 0 0 The PHOTO Trial 0 0 0 10 24 2 The PROFILE Study 0 0 0 The UK Genetics of Testicular Cancer Study 484 475 184 280 627 123 Study EM NT NE&NC Urological Tumour Group Open Studies Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

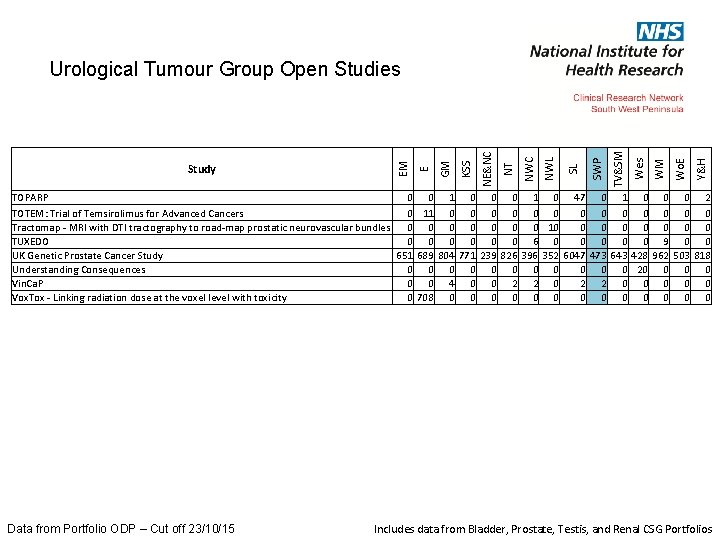
Y&H Wo. E WM Wes TV&SM SWP SL NWC NT NE&NC KSS GM E Study EM Urological Tumour Group Open Studies TOPARP 0 0 1 0 47 0 1 0 0 0 2 TOTEM: Trial of Temsirolimus for Advanced Cancers 0 11 0 0 0 0 Tractomap - MRI with DTI tractography to road-map prostatic neurovascular bundles 0 0 0 0 10 0 0 0 TUXEDO 0 0 0 6 0 0 0 9 0 0 UK Genetic Prostate Cancer Study 651 689 804 771 239 826 396 352 6047 473 643 428 962 503 818 Understanding Consequences 0 0 0 20 0 Vin. Ca. P 0 0 4 0 0 2 2 0 0 0 Vox. Tox - Linking radiation dose at the voxel level with toxicity 0 708 0 0 0 0 Data from Portfolio ODP – Cut off 23/10/15 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

Skin Tumour Group SWP Recruitment Data from Portfolio ODP – Cut off 23/10/15 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

Skin Tumour Group SWP Recruitment Trust 2014/2015/2016 Non. Commercial Total Commercial 5 23 28 2 11 13 21 35 56 6 32 38 Royal Cornwall Hospitals NHS Trust 0 75 75 2 28 30 Royal Devon and Exeter NHS Foundation Trust 0 94 94 1 86 87 Taunton and Somerset NHS Foundation Trust 23 55 78 7 29 36 Torbay and South Devon NHS Foundation Trust 8 27 35 3 14 17 Yeovil District Hospital NHS Foundation Trust 0 20 20 0 3 3 Northern Devon Healthcare NHS Trust Plymouth Hospitals NHS Trust Data from Portfolio ODP – Cut off 23/10/15 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

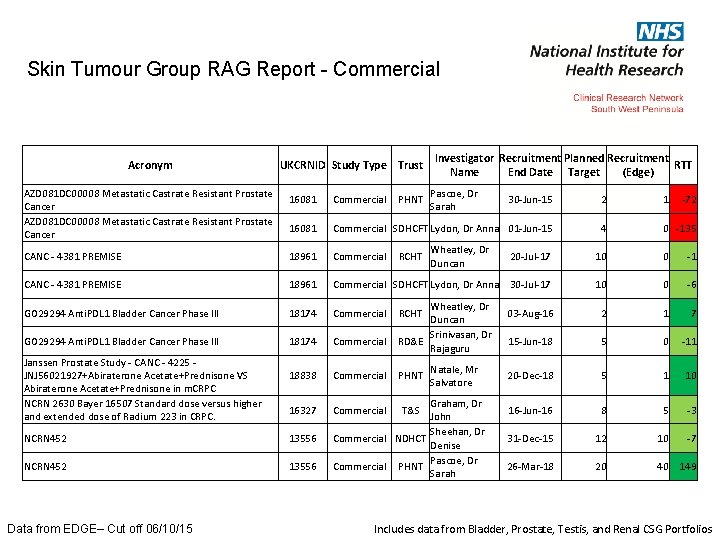
Skin Tumour Group RAG Report - Commercial Acronym AZD 081 DC 00008 Metastatic Castrate Resistant Prostate Cancer UKCRNID Study Type Trust Pascoe, Dr Sarah 16081 Commercial 30 -Jun-15 2 1 16081 Commercial SDHCFT Lydon, Dr Anna 01 -Jun-15 4 0 -135 CANC - 4381 PREMISE 18961 Commercial SDHCFT Lydon, Dr Anna GO 29294 Anti. PDL 1 Bladder Cancer Phase III 18174 Commercial 18838 Commercial 16327 Commercial Janssen Prostate Study - CANC - 4225 JNJ 56021927+Abiraterone Acetate+Prednisone VS Abiraterone Acetate+Prednisone in m. CRPC NCRN 2630 Bayer 16507 Standard dose versus higher and extended dose of Radium 223 in CRPC. NCRN 452 13556 Data from EDGE– Cut off 06/10/15 PHNT Investigator Recruitment Planned Recruitment RTT Name End Date Target (Edge) RCHT Wheatley, Dr Duncan Srinivasan, Dr RD&E Rajaguru RCHT PHNT Natale, Mr Salvatore Graham, Dr John Sheehan, Dr Commercial NDHCT Denise Pascoe, Dr Commercial PHNT Sarah T&S -72 20 -Jul-17 10 0 -1 30 -Jul-17 10 0 -6 03 -Aug-16 2 1 7 15 -Jun-18 5 0 -11 20 -Dec-18 5 1 10 16 -Jun-16 8 5 -3 31 -Dec-15 12 10 -7 26 -Mar-18 20 40 149 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

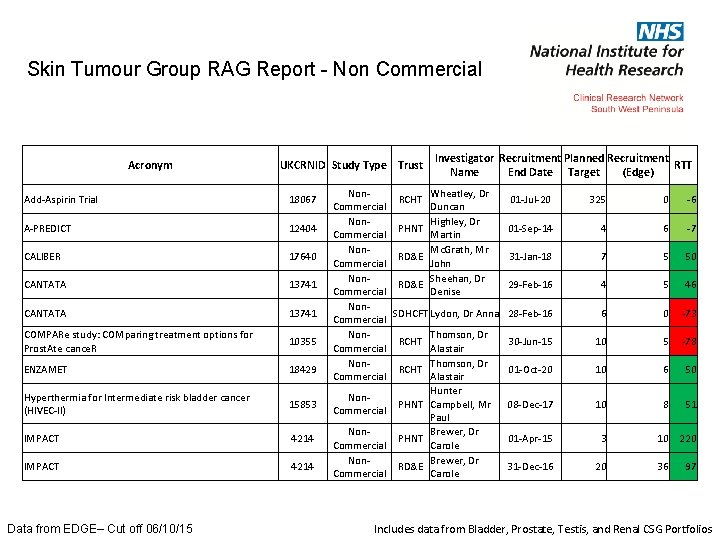
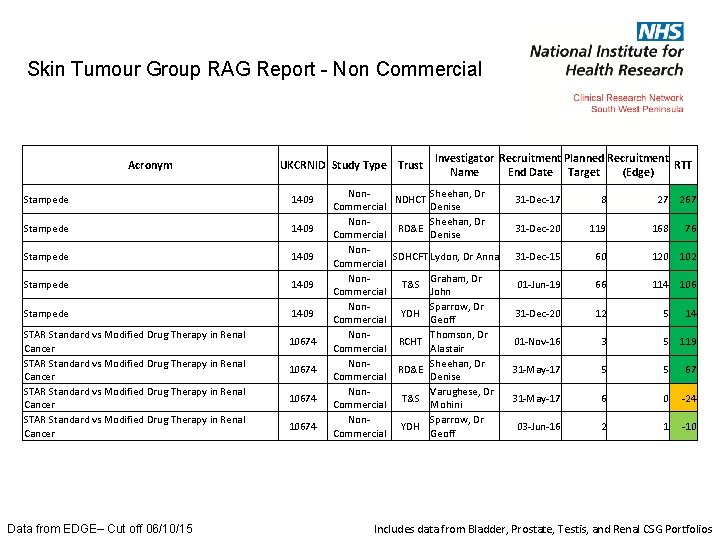
Skin Tumour Group RAG Report - Non Commercial Acronym UKCRNID Study Type Trust Add-Aspirin Trial 18067 A-PREDICT 12404 CALIBER 17640 CANTATA 13741 COMPARe study: COMparing treatment options for Prost. Ate cance. R 10355 ENZAMET 18429 Hyperthermia for Intermediate risk bladder cancer (HIVEC-II) 15853 IMPACT 4214 Data from EDGE– Cut off 06/10/15 Investigator Recruitment Planned Recruitment RTT Name End Date Target (Edge) Non. Wheatley, Dr RCHT Commercial Duncan Non. Highley, Dr PHNT Commercial Martin Non. Mc. Grath, Mr RD&E Commercial John Non. Sheehan, Dr RD&E Commercial Denise Non. SDHCFT Lydon, Dr Anna Commercial Non. Thomson, Dr RCHT Commercial Alastair Hunter Non. PHNT Campbell, Mr Commercial Paul Non. Brewer, Dr PHNT Commercial Carole Non. Brewer, Dr RD&E Commercial Carole 01 -Jul-20 325 0 -6 01 -Sep-14 4 6 -7 31 -Jan-18 7 5 50 29 -Feb-16 4 5 46 28 -Feb-16 6 0 -73 30 -Jun-15 10 5 -78 01 -Oct-20 10 6 50 08 -Dec-17 10 8 51 01 -Apr-15 3 31 -Dec-16 20 10 220 36 97 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

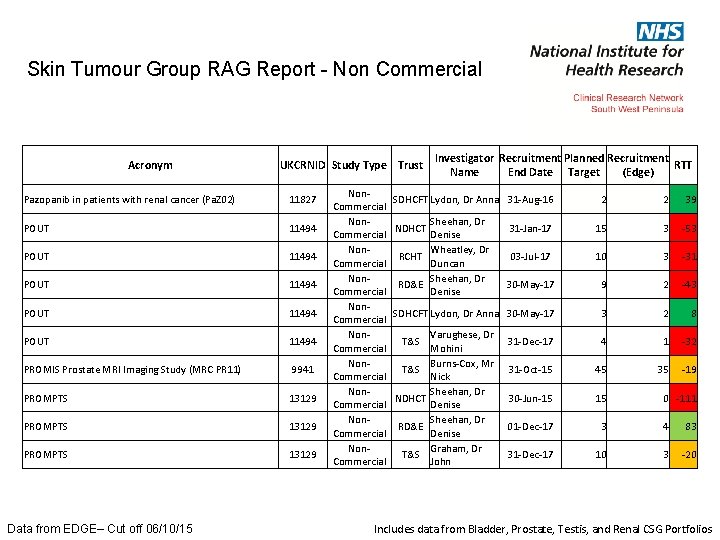
Skin Tumour Group RAG Report - Non Commercial Acronym UKCRNID Study Type Trust Pazopanib in patients with renal cancer (Pa. Z 02) 11827 POUT 11494 POUT 11494 PROMIS Prostate MRI Imaging Study (MRC PR 11) 9941 PROMPTS 13129 Data from EDGE– Cut off 06/10/15 Non. Commercial Non. Commercial Investigator Recruitment Planned Recruitment RTT Name End Date Target (Edge) SDHCFT Lydon, Dr Anna 31 -Aug-16 2 2 39 31 -Jan-17 15 3 -53 03 -Jul-17 10 3 -31 30 -May-17 9 2 -43 SDHCFT Lydon, Dr Anna 30 -May-17 3 2 8 31 -Dec-17 4 1 -32 31 -Oct-15 45 35 -19 30 -Jun-15 15 01 -Dec-17 3 4 83 31 -Dec-17 10 3 -20 Sheehan, Dr Denise Wheatley, Dr RCHT Duncan Sheehan, Dr RD&E Denise NDHCT T&S NDHCT RD&E T&S Varughese, Dr Mohini Burns-Cox, Mr Nick Sheehan, Dr Denise Graham, Dr John 0 -111 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

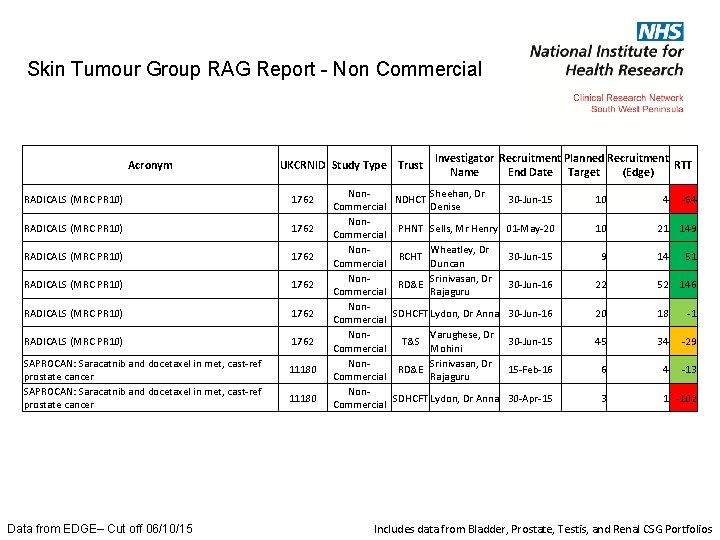
Skin Tumour Group RAG Report - Non Commercial Acronym UKCRNID Study Type Trust RADICALS (MRC PR 10) 1762 RADICALS (MRC PR 10) 1762 SAPROCAN: Saracatnib and docetaxel in met, cast-ref prostate cancer Data from EDGE– Cut off 06/10/15 11180 Investigator Recruitment Planned Recruitment RTT Name End Date Target (Edge) Non. Sheehan, Dr NDHCT 30 -Jun-15 Commercial Denise Non. PHNT Sells, Mr Henry 01 -May-20 Commercial Non. Wheatley, Dr RCHT 30 -Jun-15 Commercial Duncan Non. Srinivasan, Dr RD&E 30 -Jun-16 Commercial Rajaguru Non. SDHCFT Lydon, Dr Anna 30 -Jun-16 Commercial Non. Varughese, Dr T&S 30 -Jun-15 Commercial Mohini Non. Srinivasan, Dr RD&E 15 -Feb-16 Commercial Rajaguru Non. SDHCFT Lydon, Dr Anna 30 -Apr-15 Commercial 10 10 9 4 -64 21 149 14 51 22 52 146 20 18 -1 45 34 -29 6 4 -13 3 1 -102 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

Skin Tumour Group RAG Report - Non Commercial Acronym UKCRNID Study Type Trust Stampede 1409 Stampede 1409 STAR Standard vs Modified Drug Therapy in Renal Cancer Data from EDGE– Cut off 06/10/15 10674 Investigator Recruitment Planned Recruitment RTT Name End Date Target (Edge) Non. Sheehan, Dr NDHCT Commercial Denise Non. Sheehan, Dr RD&E Commercial Denise Non. SDHCFT Lydon, Dr Anna Commercial Non. Graham, Dr T&S Commercial John Non. Sparrow, Dr YDH Commercial Geoff Non. Thomson, Dr RCHT Commercial Alastair Non. Sheehan, Dr RD&E Commercial Denise Non. Varughese, Dr T&S Commercial Mohini Non. Sparrow, Dr YDH Commercial Geoff 31 -Dec-17 8 27 267 31 -Dec-20 119 168 76 31 -Dec-15 60 120 102 01 -Jun-19 66 114 106 31 -Dec-20 12 5 14 01 -Nov-16 3 5 119 31 -May-17 5 5 67 31 -May-17 6 0 -24 03 -Jun-16 2 1 -10 Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios

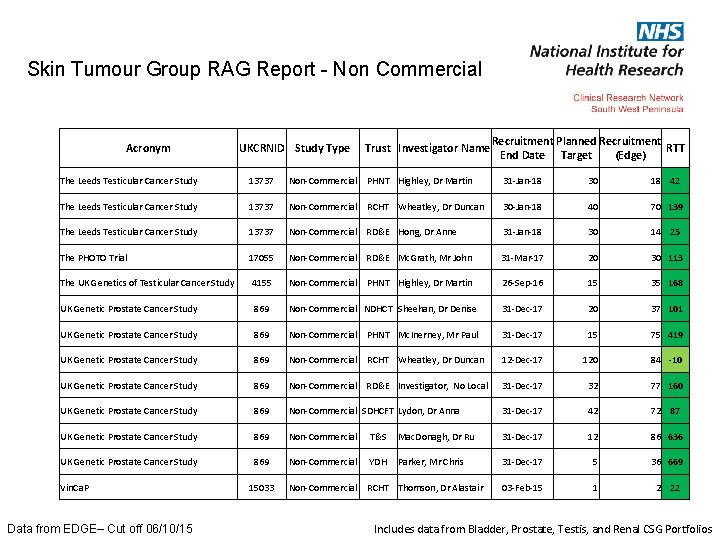
Skin Tumour Group RAG Report - Non Commercial Acronym UKCRNID Study Type Trust Investigator Name Recruitment Planned Recruitment RTT End Date Target (Edge) The Leeds Testicular Cancer Study 13737 Non-Commercial PHNT Highley, Dr Martin 31 -Jan-18 30 18 42 The Leeds Testicular Cancer Study 13737 Non-Commercial 30 -Jan-18 40 70 139 The Leeds Testicular Cancer Study 13737 Non-Commercial RD&E Hong, Dr Anne 31 -Jan-18 30 14 25 The PHOTO Trial 17055 Non-Commercial RD&E Mc. Grath, Mr John 31 -Mar-17 20 30 113 The UK Genetics of Testicular Cancer Study 4155 Non-Commercial PHNT Highley, Dr Martin 26 -Sep-16 15 35 168 UK Genetic Prostate Cancer Study 869 Non-Commercial NDHCT Sheehan, Dr Denise 31 -Dec-17 20 37 101 UK Genetic Prostate Cancer Study 869 Non-Commercial PHNT Mc. Inerney, Mr Paul 31 -Dec-17 15 75 419 UK Genetic Prostate Cancer Study 869 Non-Commercial 12 -Dec-17 120 84 -10 UK Genetic Prostate Cancer Study 869 Non-Commercial RD&E Investigator, No Local 31 -Dec-17 32 77 160 UK Genetic Prostate Cancer Study 869 Non-Commercial SDHCFT Lydon, Dr Anna 31 -Dec-17 42 72 87 UK Genetic Prostate Cancer Study 869 Non-Commercial T&S Mac. Donagh, Dr Ru 31 -Dec-17 12 86 636 UK Genetic Prostate Cancer Study 869 Non-Commercial YDH Parker, Mr Chris 31 -Dec-17 5 36 669 15033 Non-Commercial RCHT Thomson, Dr Alastair 03 -Feb-15 1 2 22 Vin. Ca. P Data from EDGE– Cut off 06/10/15 RCHT Wheatley, Dr Duncan Includes data from Bladder, Prostate, Testis, and Renal CSG Portfolios
 Giant cell tumour
Giant cell tumour Adenoma
Adenoma Brain tumors
Brain tumors Portfolio sample for students
Portfolio sample for students Portfolio perspective
Portfolio perspective One point perspective boxes
One point perspective boxes Silo perspective vs business process perspective
Silo perspective vs business process perspective National 5 pe portfolio marking scheme
National 5 pe portfolio marking scheme Portfolio solutions group
Portfolio solutions group Dod taxonomy
Dod taxonomy National unification and the national state
National unification and the national state National focus group
National focus group National business group on health conference 2018
National business group on health conference 2018 National working group on swiss franc reference rates
National working group on swiss franc reference rates Advisory expert group on national accounts
Advisory expert group on national accounts Switzerland starter pack
Switzerland starter pack