URINE ANALYSIS 1 Clinical Significance Monitoring of kidney

















- Slides: 21

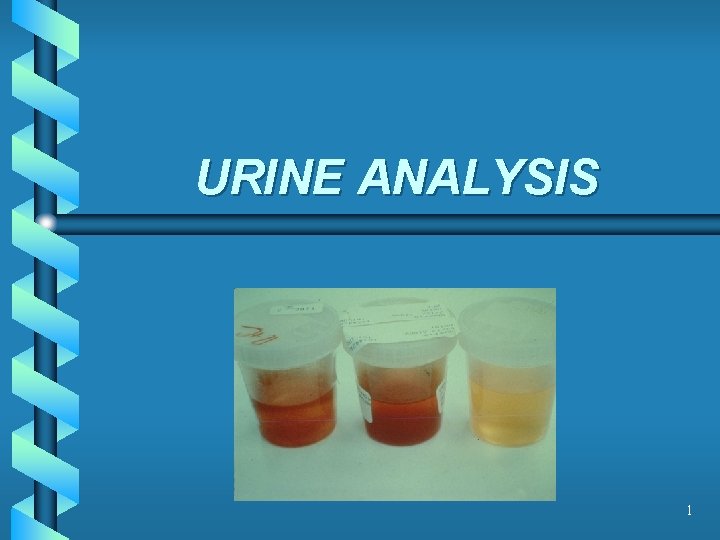
URINE ANALYSIS 1

Clinical Significance Monitoring of kidney disease. Checking for UTI. Checking for hematuria. Monitoring of diabetes. 2

Preparation of Patient Child and Adult: C-C MSU Infant 3

Clean Catch Midstream Bacteria in the urethra. Used for: routine urinalysis, culture, or other tests. 4

Collection of Specimen Random First morning specimen 5

Storage Considerations Protected from direct sunlight. Fresh & free from preservatives. Analyzed within 1 hr of voiding. If not possible, store in the dark at 280 C to preserve chemical & cellular constituents. Refrigeration may increase the precipitation of amorphous & crystalline materials. 6

Color Performed on well-mixed, un-centrifuged specimen. Normal: Varies according to the concentration of urine (fluid intake). Abnormal: Pathologic conditions, medications or diet. Microscopy & chemical analysis usually identify the cause. 7

Appearance Indicates the presence of particulate matter. Normal urine is essentially clear; although cloudy urine does not necessarily indicate a pathologic condition. 8

Chemical Examination of Urine- Dipstick A specific chemical reaction is combined with an indicator or conjugated reagent to produce a color of varying intensity proportional to the tested analyte concentration. Test qualitatively or semi-quantitatively between 1 & 11 urine parameters. Only freshly voided urine that has not been centrifuged should be used. 9

Special Considerations Any color changes appearing only along the edges of the test patches, or developing after the time of reading do not have any diagnostic significance. Any questionable reactions with the dipsticks should be checked with the confirmatory tests. 10

Specific Gravity Amount of dissolved solids in urine or its ion concentration. Reflects the ability of the kidney to concentrate and dilute urine. 11

Refractometer Measures the RI of a solution (velocity of light in air: velocity of light in a solution). The velocity depends on the number of dissolved particles in the solution & determines the angle at which light passes through the solution. The refractometer measures the angle & mathematically converts this angle to SG. Very concentrated specimens need to be diluted. 12

Microscopic Examination The conventional method: cells/HPF. The counting chamber: quantitation. Chamber counts on centrifuged urine sediments are more reliable in predicting renal functional abnormalities than using cells /HPF. The cells from undiluted well-mixed & centrifuged urine are counted & reported as the number of cells x 106/L. 13

Recommendations on Urinary Sediment Abnormal Findings More than two renal epithelial cells / HPF. Pathologic casts. Atypical mononuclear cells. Tissue fragments. Pathologic crystals. Referred: reported, underlined & brought to the attention of the physician, confirmed with specialized testing (cytopathologic examination or specific chemical tests). 14

QA Failure to maintain the identity of a specimen throughout the testing stages. Failure to follow recommended procedures of the laboratory & manufacturer regarding both reagents & control material. Lack of good control procedures. Lack of experience and training. 15

QC ID, labeling & handling. Proper collection (CC-MSU, clean container, no visible signs of contamination). Collection time & no delays in transportation. Performance of refractometer, dipsticks microscope, & all test methods must be compared with QC materials. 16

QC No reagents or controls must be used beyond their expiration dates, even if they seem to be functioning properly. Any reagent that looks unusual or reacts outside of the reference or control range should not be used. If a QC result is out of the acceptable limits, the problem must be identified & corrected before any patient testing can be performed. 17

QC of Refractometer SG of known substances should be measured and the results should be recorded. SG of a normal & abnormal control substance should be measured & the results recorded. 18

QC of Dipsticks are sensitive to moisture, heat & light exposure. Store in a cool & dry place. Discolored strips: contamination. Container recapped after use. The chemical testing areas should not be touched. Dipsticks should be checked each day of use with at least 2 levels of a control 19 material.

QC of Dipsticks New container of strips or a new control: strips tested with the control material. All lot numbers & expiration dates should be recorded. Any questionable reactions checked with the confirmatory tests. (Interfering substances). Each person involved in the reading of macroscopic reagent strips should be 20 examined for color blindness.

QC of Urinary Sediment Commercially available control materials can be used. The package insert lists the expected ranges for the elements that should be found in the sediment of the control. The microscope function should be monitored; lenses, objectives and filters should all be clean. The counting chamber and cover slip should be clean and properly calibrated. 21