Upstream Process Fermentation Batch Culture Part I WarilaCCRI
Upstream Process Fermentation Batch Culture Part I Warila-CCRI

Microbial Growth Phases (Fermentation) Lag Phase Exponential Phase Stationary Phase Death Phase
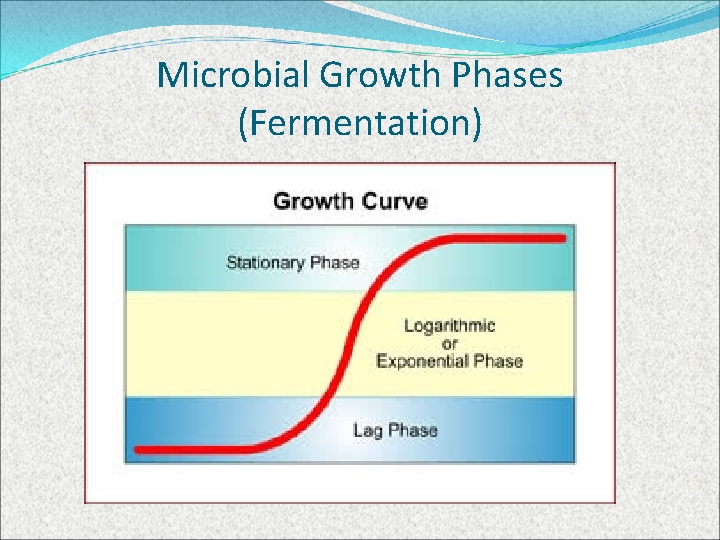
Microbial Growth Phases (Fermentation)
Lag Phase The first phase of growth in a batch culture A period of adaptation of the cells to their new environment Little increase in cell density May not last long in some fermentations

Exponential Phase Also known as the logarithmic growth phase Cells are adjusted to their environment and are dividing continuously results in an exponential increase in the number of cells Cell growth rate is often substrate limited
Stationary Phase When the number of cells dividing and dying is the same Depletion of one or more essential growth nutrients Accumulation of toxic by-products Stress associated with the induction of a recombinant gene

Stationary Phase Primary growth associated production stops Secondary, non-growth associated, production may continue This where the product is produced ($$$) The stationary phase can also be induced chemically - Sodium butyrate is an example of such a chemical

Death Phase Also known as the decline phase The rate of cells dying is greater than the rate of cells dividing

Establishing a Growth Curve – Counting the Cells Viable (living) Cell Count Optical Density

Viable (living) Cell Count represents the cells are actually living (more accurate) Counts made using a hemocytometer /trypan blue stain under a microscope

Viable (living) Cell Count or plating a sample from the culture and counting colonies

Optical Density (turbidity, absorbance) Counts made by taking an optical measurement using a spectrophotometer Measures living and dead cells (less accurate) Different cell types are measured at different wavelengths (i. e. E. coli @ 600 nm) The measurements are compared to reference readings made previously

Spectrophotometers New Model Old Model UV Vis Light

Spectrophotometers • Useful for trace analysis • Utilizes the Lambert-Beer Law: Ølogarithmic relationship of the absorption of light in relation to the concentration of the material it is travelling through

Spectrophotometers • Light spectrophotometers can be dialed in to a desired wavelength for a sample (400700 nm) • Light of this wavelength then passes through the sample • The intensity of the light that passes through the sample is then measured by a sensor or detector at the other end

Spectrophotometers • This intensity is translated in a number by the spectrophotometer • This number can be read as transmittance or absorbance • We like to use absorbance as it gives a linear curve (straight line)

Spectrophotometers • The instrument should be “zeroed” or “blanked” with a cuvette containing only a clean sample of the substance • This will serve as a baseline to which other standards or samples will be compared

- Slides: 18