Upper GI Cancers Risk Stratification and Treatment Selection













- Slides: 59

Upper GI Cancers: Risk Stratification and Treatment Selection David H. Ilson, MD, Ph. D Gastrointestinal Oncology Service Memorial Sloan-Kettering Cancer Center

Disclosure l Research Funding – Roche-Genentech – Bayer – sanofi-aventis – BMS-Imclone

UGI Cancers, Risk Stratification and Therapy l Staging of Gastric and Esophageal Cancer for treatment selection l Benefits of adjuvant chemotherapy and radiation therapy l Appropriate selection of chemotherapy for Stage IV disease l Pancreatic Cancer adjuvant and advanced disease therapy

Esophageal and Gastric Carcinoma US Incidence in 2011 l 38, 500 new cases l Decline in Gastric Cancer Incidence l Increase in Esophageal , GE JX, cardia adeno l OS improvement, 1975 -77, 1984 -86, 1999 -2006 – Gastric: 16% 18% 27% – Esophageal: 5% 10% 19% l Highly virulent diseases with poor outcome Jemal et al, CA 61: 212 -236; 2011

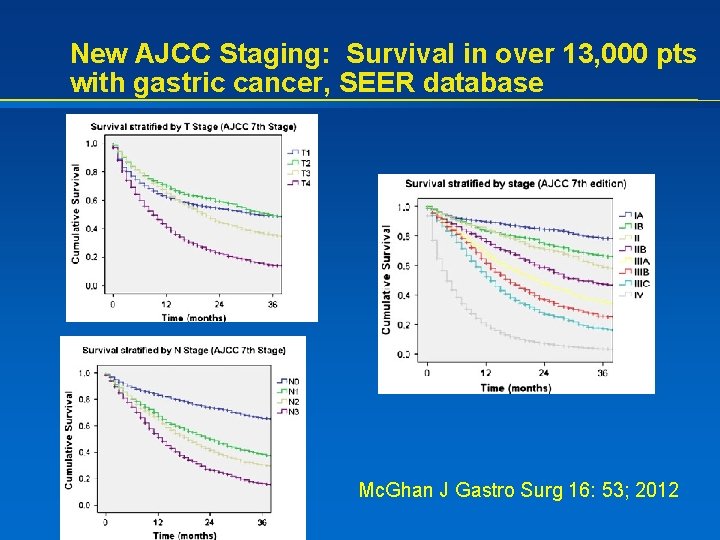
New AJCC Staging: Survival in over 13, 000 pts with gastric cancer, SEER database Mc. Ghan J Gastro Surg 16: 53; 2012

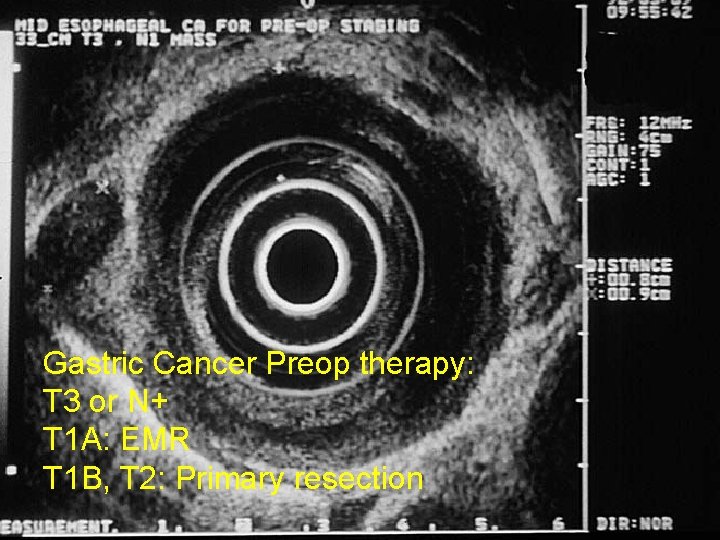
Gastric Cancer Preop therapy: T 3 or N+ T 1 A: EMR T 1 B, T 2: Primary resection

New AJCC Staging: Survival in over 4600 pts with esophageal and GEJ cancer Rice Cancer 2010

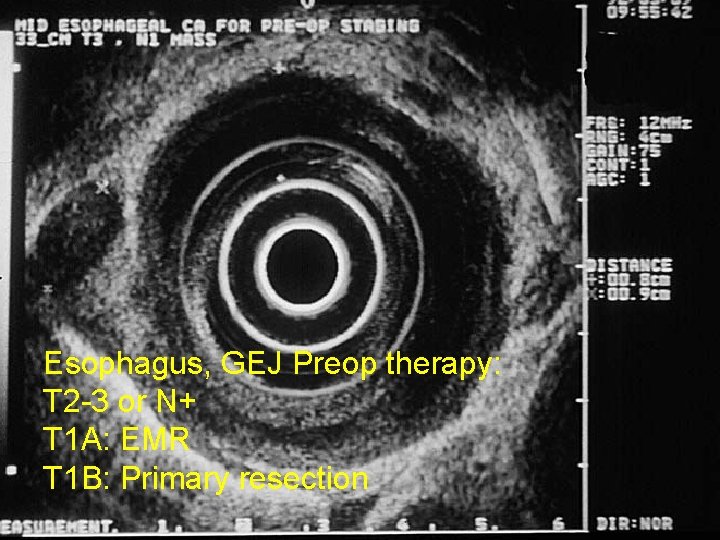
Esophagus, GEJ Preop therapy: T 2 -3 or N+ T 1 A: EMR T 1 B: Primary resection

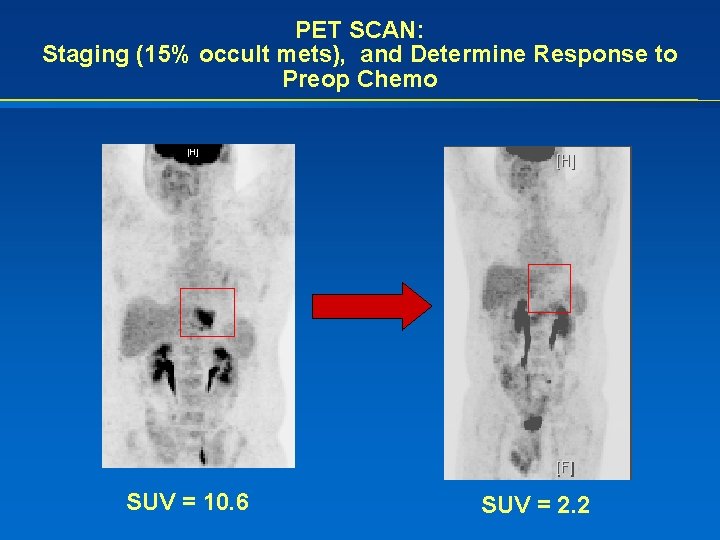
PET SCAN: Staging (15% occult mets), and Determine Response to Preop Chemo SUV = 10. 6 SUV = 2. 2

Laparoscopy in Gastric Cancer l CT and PET scan may miss small volume liver or peritoneal disease l For gastric cancer, laparoscopy detects peritoneal or liver disease in 20 -30% of patients – Not mandated for GEJ cancers: < 5% positive lap findings l A positive cytology = Stage IV disease – Patients do not benefit from immediate gastrectomy – They should be treated with palliative chemotherapy – ? Reassess response and consider selective surgery n No long term survivors with + cytology

Adjuvant Therapy in Gastric Cancer Improves OS l Pre and post op chemo (U. K. ) – ECF, MAGIC: n 13% 5 yr OS, HR 0. 75 l Post op chemo (Asia): 2 trials, 2000 pts, D 2 resection – S-1, ACTS-GC: n 13% 5 yr OS, HR 0. 67 (2011 update) – Post op Cape-Oxali , CLASSIC Trial: n 14% 3 yr DFS, HR 0. 56 l Post op RT + chemo (U. S. ), less than a D 1 -2 resection – 5 FU-LV + RT, INT 116: n 10% 5 yr OS, HR 0. 65 Cunningham NEJM 355: 11; 2006 Sasako JCO 29: 4387; 2011 Bang LBA 4002, Proc ASCO 2011 Macdonald NEJM 345: 725; 2001

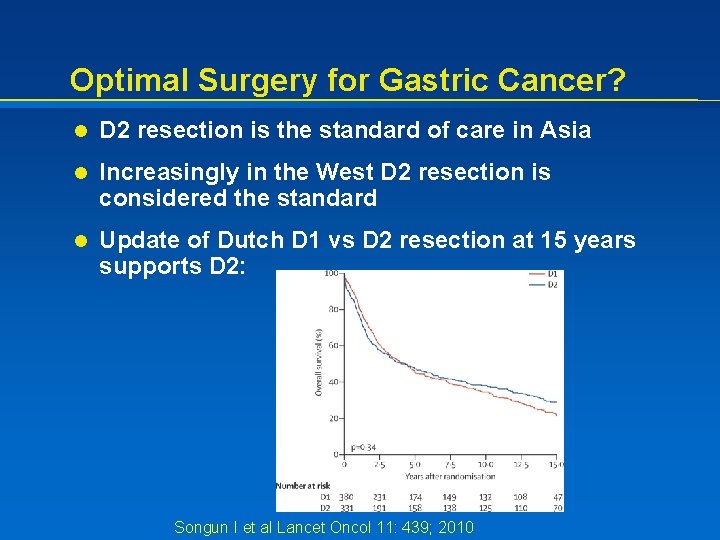
Optimal Surgery for Gastric Cancer? l D 2 resection is the standard of care in Asia l Increasingly in the West D 2 resection is considered the standard l Update of Dutch D 1 vs D 2 resection at 15 years supports D 2: Songun I et al Lancet Oncol 11: 439; 2010

Optimal Adjuvant Chemotherapy? l Support of 5 -FU monotherapy – ACTS-GC: S-1 – CALGB 80101: n ECF no better than 5 -FU/LV, when given post op with FU + RT l Support for 5 -FU + platinum agent – CLASSIC: Capecitabine-Oxaliplatin – FNCLCC-FFCD and MAGIC: CF and ECF Fuchs Abs 4003, Proc ASCO 2011 Bang LBA 4002, Proc ASCO 2011 Ychou J Clin Oncol 29: 1715; 2011

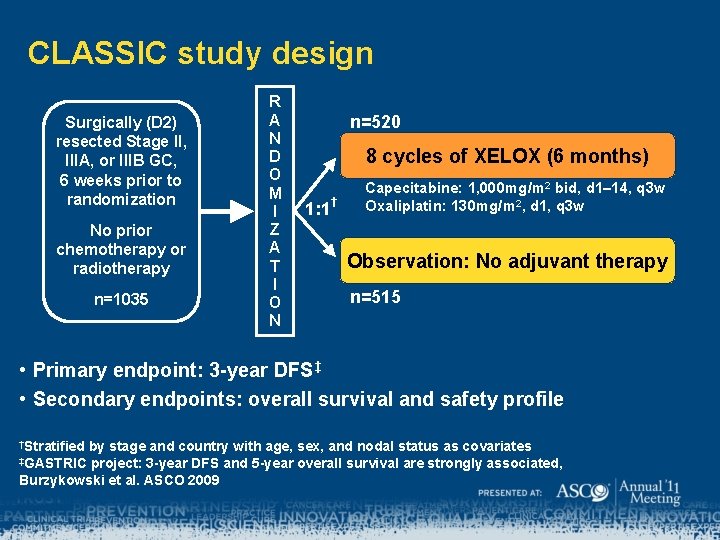
CLASSIC study design Surgically (D 2) resected Stage II, IIIA, or IIIB GC, 6 weeks prior to randomization No prior chemotherapy or radiotherapy n=1035 R A N D O M I Z A T I O N n=520 8 cycles of XELOX (6 months) 1: 1 † Capecitabine: 1, 000 mg/m 2 bid, d 1– 14, q 3 w Oxaliplatin: 130 mg/m 2, d 1, q 3 w Observation: No adjuvant therapy n=515 • Primary endpoint: 3 -year DFS‡ • Secondary endpoints: overall survival and safety profile †Stratified by stage and country with age, sex, and nodal status as covariates project: 3 -year DFS and 5 -year overall survival are strongly associated, Burzykowski et al. ASCO 2009 ‡GASTRIC

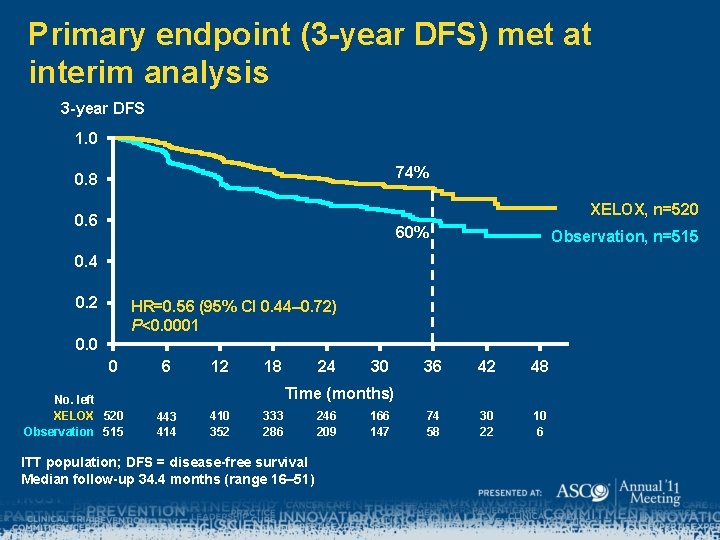
Primary endpoint (3 -year DFS) met at interim analysis 3 -year DFS 1. 0 74% 0. 8 XELOX, n=520 0. 6 60% Observation, n=515 0. 4 0. 2 HR=0. 56 (95% CI 0. 44– 0. 72) P<0. 0001 0. 0 0 No. left XELOX 520 Observation 515 6 12 18 24 30 36 42 48 74 58 30 22 10 6 Time (months) 443 414 410 352 333 286 ITT population; DFS = disease-free survival Median follow-up 34. 4 months (range 16– 51) 246 209 166 147

Regional Therapies as Adjuvant? l Role of post op RT – U. S. INT 116: < D 1 -2 resection, RT reduced local recurrence – ARTIST (Korea, JCO in press), D 2 resection n Cape-Cis vs Cape-Cis + RT n DFS benefit in node + patients for adding RT § 5% improvement in 3 year DFS, HR 0. 69 l Ongoing Trials – CRITICS: n Preop ECX, post op ECX + / - RT – TOPGEAR: n Preop ECX + / - RT

Esophageal Adenocarcinoma: Adjuvant Therapy Improves OS l T 2 -3 or N 1: Something more than surgery alone should be done l Preoperative chemotherapy ECF, CF improves overall survival in some but not all trials – MAGIC (ECF): 13% ↑ OS at 5 yr (75% gastric, 25% esophageal) – FFCD / FNLC (CF): 14% ↑ OS at 5 yr (gastric and esophageal cancer) same as MAGIC, no epirubicin – MRC 0 E 0 -2 (CF): Esophageal n 5 year update: 6%, no impact on distant recurrence – U. S. INT 113 (CF): no impact on OS – EORTC 40954 (CF): no impact on OS MRC Lancet 359: 1727; 2002 Cunningham NEJM 355: 11; 2006 Schumacher JCO 28: 5210; 2010

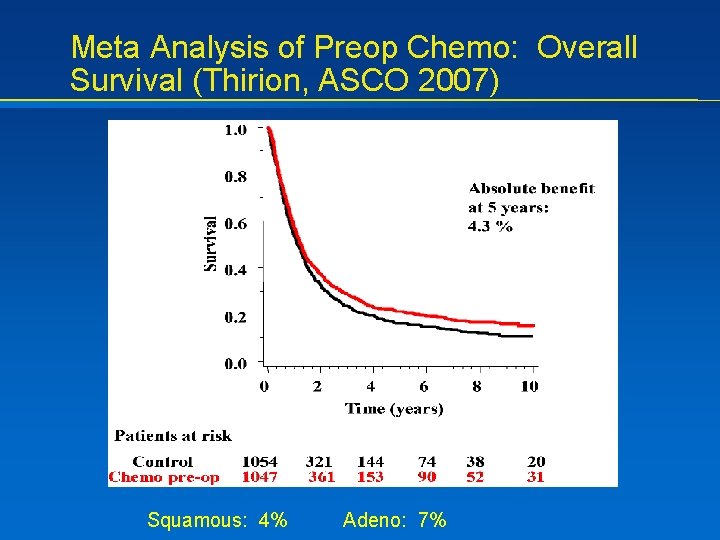
Meta Analysis of Preop Chemo: Overall Survival (Thirion, ASCO 2007) Squamous: 4% Adeno: 7%

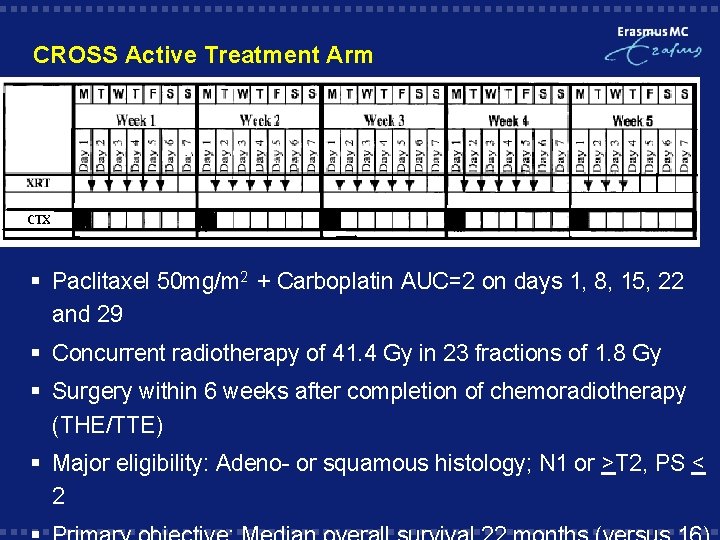
CROSS Active Treatment Arm § Paclitaxel 50 mg/m 2 + Carboplatin AUC=2 on days 1, 8, 15, 22 and 29 § Concurrent radiotherapy of 41. 4 Gy in 23 fractions of 1. 8 Gy § Surgery within 6 weeks after completion of chemoradiotherapy (THE/TTE) § Major eligibility: Adeno- or squamous histology; N 1 or >T 2, PS < 2

CROSS: Major Results • • EUS staged patients T 3 N 0 -1 75%, median age 60 74% Adenocarcinoma 93% received all courses chemotherapy – 23% had > = grade 3 toxicity from pre-op therapy • Post-operative morbidity and mortality almost identical (mortality 3. 7 -3. 8%) • Path CR rate of nearly 30% with chemo RT 20

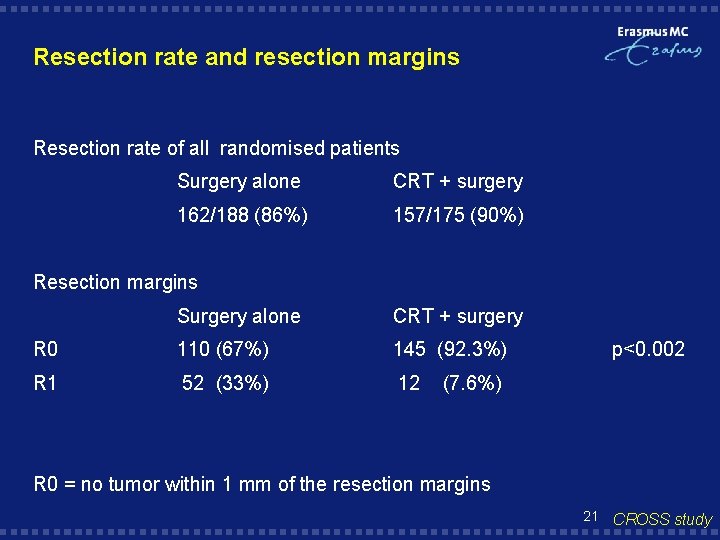
Resection rate and resection margins Resection rate of all randomised patients Surgery alone CRT + surgery 162/188 (86%) 157/175 (90%) Resection margins Surgery alone CRT + surgery R 0 110 (67%) 145 (92. 3%) R 1 52 (33%) 12 p<0. 002 (7. 6%) R 0 = no tumor within 1 mm of the resection margins 21 CROSS study

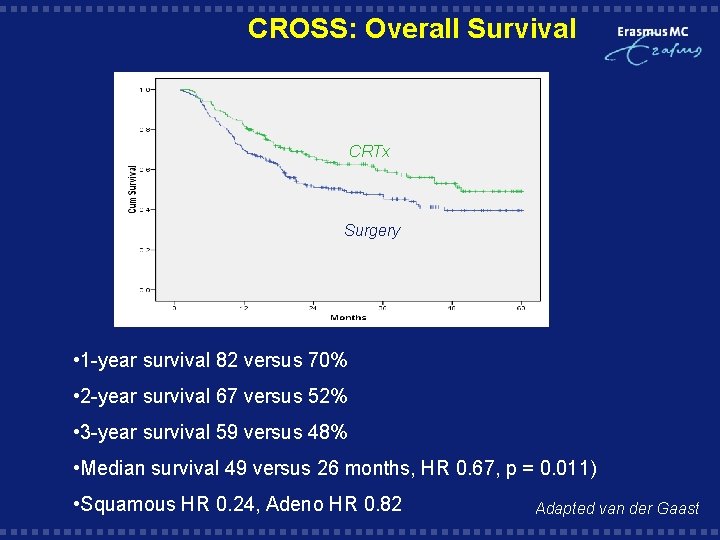
CROSS: Overall Survival CRTx Surgery HR 0. 67 95% CI (. 49 -. 91) P=0. 012 HR 0. 67 95% CI (0. 49 - 0. 91) • 1 -year survival 82 versus 70% • 2 -year survival 67 versus 52% • 3 -year survival 59 versus 48% • Median survival 49 versus 26 months, HR 0. 67, p = 0. 011) • Squamous HR 0. 24, Adeno HR 0. 82 Adapted van der Gaast

Preop Chemo vs Chemo. RT

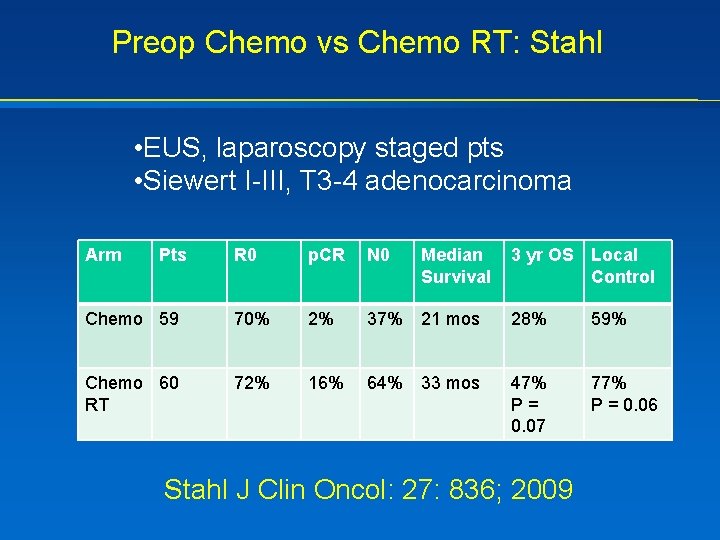
Preop Chemo vs Chemo RT: Stahl • EUS, laparoscopy staged pts • Siewert I-III, T 3 -4 adenocarcinoma Arm Pts R 0 p. CR N 0 Median Survival 3 yr OS Local Control Chemo 59 70% 2% 37% 21 mos 28% 59% Chemo 60 RT 72% 16% 64% 33 mos 47% P= 0. 07 77% P = 0. 06 Stahl J Clin Oncol: 27: 836; 2009

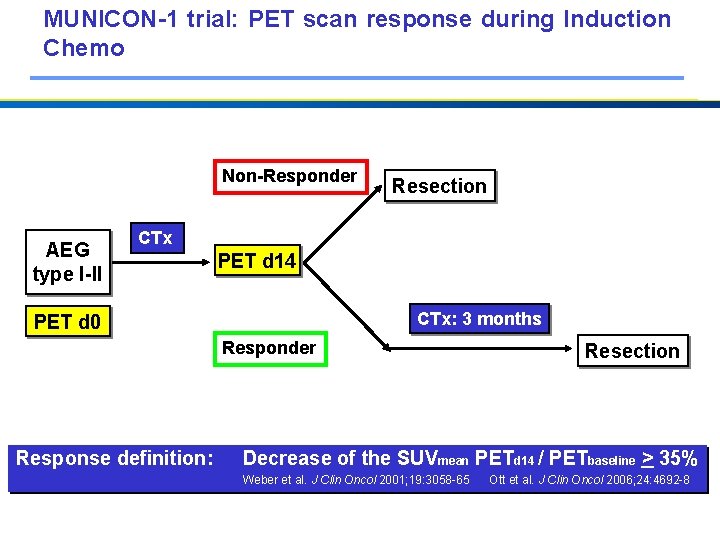
MUNICON-1 trial: PET scan response during Induction Chemo Non-Responder AEG type I-II Resection CTx PET d 14 CTx: 3 months PET d 0 Responder Response definition: Resection Decrease of the SUVmean PETd 14 / PETbaseline > 35% Weber et al. J Clin Oncol 2001; 19: 3058 -65 Ott et al. J Clin Oncol 2006; 24: 4692 -8

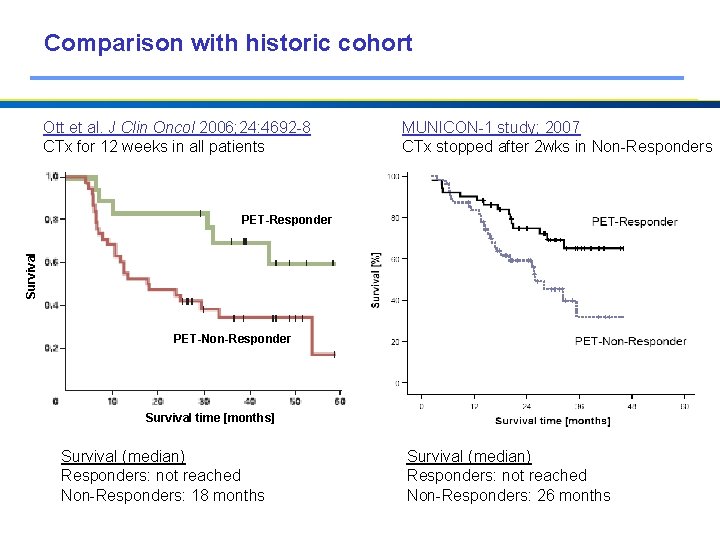
Comparison with historic cohort Ott et al. J Clin Oncol 2006; 24: 4692 -8 CTx for 12 weeks in all patients MUNICON-1 study; 2007 CTx stopped after 2 wks in Non-Responders Survival PET-Responder PET-Non-Responder Survival time [months] Survival (median) Responders: not reached Non-Responders: 18 months Survival (median) Responders: not reached Non-Responders: 26 months

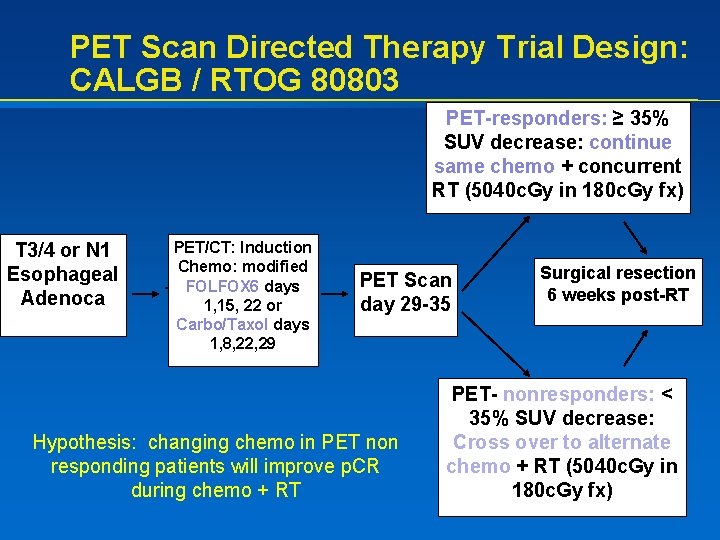
PET Scan Directed Therapy Trial Design: CALGB / RTOG 80803 PET-responders: ≥ 35% SUV decrease: continue same chemo + concurrent RT (5040 c. Gy in 180 c. Gy fx) T 3/4 or N 1 Esophageal Adenoca PET/CT: Induction Chemo: modified FOLFOX 6 days 1, 15, 22 or Carbo/Taxol days 1, 8, 22, 29 PET Scan day 29 -35 Hypothesis: changing chemo in PET non responding patients will improve p. CR during chemo + RT Surgical resection 6 weeks post-RT PET- nonresponders: < 35% SUV decrease: Cross over to alternate chemo + RT (5040 c. Gy in 180 c. Gy fx)

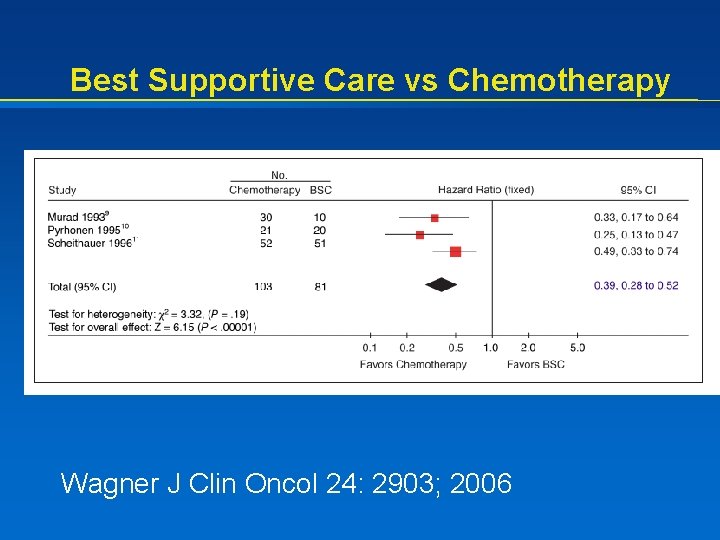
Best Supportive Care vs Chemotherapy Wagner J Clin Oncol 24: 2903; 2006

Advanced Gastric Cancer Chemotherapy: What regimen to use? Oxali: Cape: XP FLO FUFIRI S-1 Cis DCF EOX or EOF ECX or EOX Pts 489 513 160 109 170 305 221 126 %RR 44% 45% 41% 34% 32% 54% 36% 45% TTP, mos 6. 7 6. 5 5. 6 5. 5 5. 0 6. 0 5. 6 7. 4 OS, mos 10. 4 10. 5 -- 9. 0 13. 0 9. 2 8. 9 10. 9

Patient Selection for Chemotherapy l Assess age, functional status, comorbidites l Combination chemotherapy preferred over single agents – Monotherapy with 5 -FU, capecitabine, taxanes in elderly, poor PS patients l 3 drug regimens (DCF, m. DCF) – High functional status, younger patients without comorbidities – Willingness to tolerate side effects – Access to frequent follow up and toxicity assessment

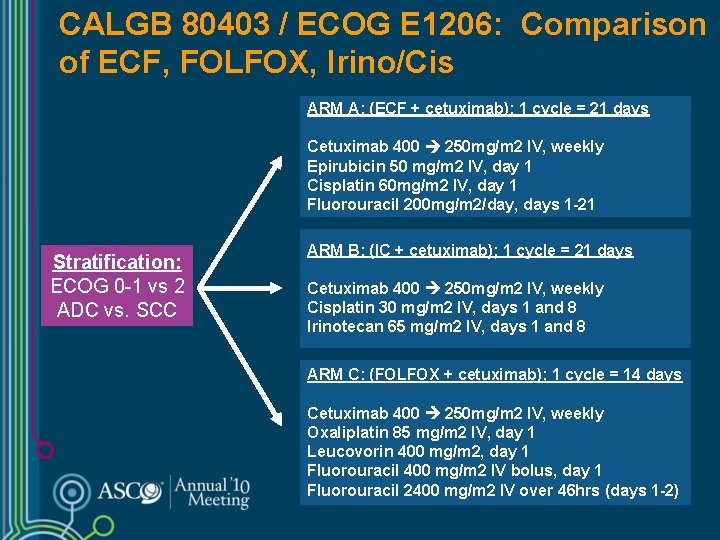
CALGB 80403 / ECOG E 1206: Comparison of ECF, FOLFOX, Irino/Cis ARM A: (ECF + cetuximab); 1 cycle = 21 days Cetuximab 400 250 mg/m 2 IV, weekly Epirubicin 50 mg/m 2 IV, day 1 Cisplatin 60 mg/m 2 IV, day 1 Fluorouracil 200 mg/m 2/day, days 1 -21 Stratification: ECOG 0 -1 vs 2 ADC vs. SCC ARM B: (IC + cetuximab); 1 cycle = 21 days Cetuximab 400 250 mg/m 2 IV, weekly Cisplatin 30 mg/m 2 IV, days 1 and 8 Irinotecan 65 mg/m 2 IV, days 1 and 8 ARM C: (FOLFOX + cetuximab); 1 cycle = 14 days Cetuximab 400 250 mg/m 2 IV, weekly Oxaliplatin 85 mg/m 2 IV, day 1 Leucovorin 400 mg/m 2, day 1 Fluorouracil 400 mg/m 2 IV bolus, day 1 Fluorouracil 2400 mg/m 2 IV over 46 hrs (days 1 -2)

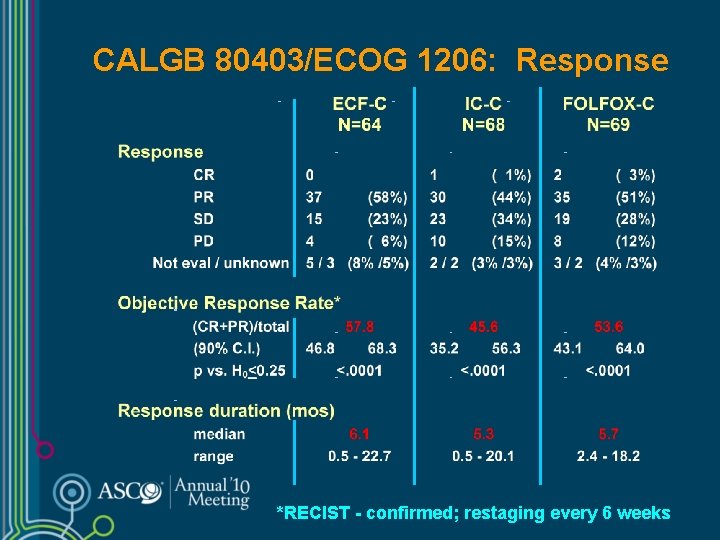
CALGB 80403/ECOG 1206: Response *RECIST - confirmed; restaging every 6 weeks

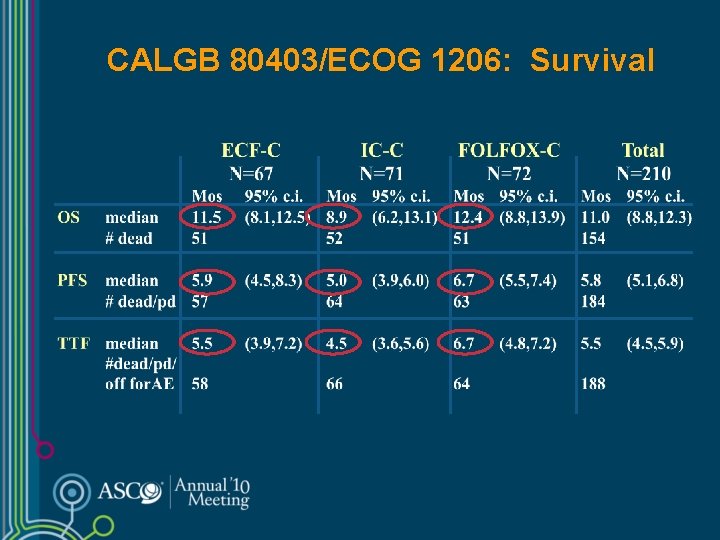
CALGB 80403/ECOG 1206: Survival

CALGB 80403: Esophageal, GE Junction Cancers l Phase II trial of Chemo + Cetuximab l FOLFOX behaved as well as ECF with less toxicity – Irinotecan and cisplatin had lowest efficacy and highest toxicity l Optimal irinotecan combination? – Irinotecan + cisplatin; significant second line activity – First Line: Irinotecan + infusional 5 -FU preferred

Colorectal Style Chemotherapy and Gastric Cancer l Both FOLFOX and FOLFIRI like regimens have acceptable activity in gastric cancer – Can be considered first line therapy l Toxicity profiles favor these regimens over conventional high dose cisplatin + 5 day infusion 5 -FU

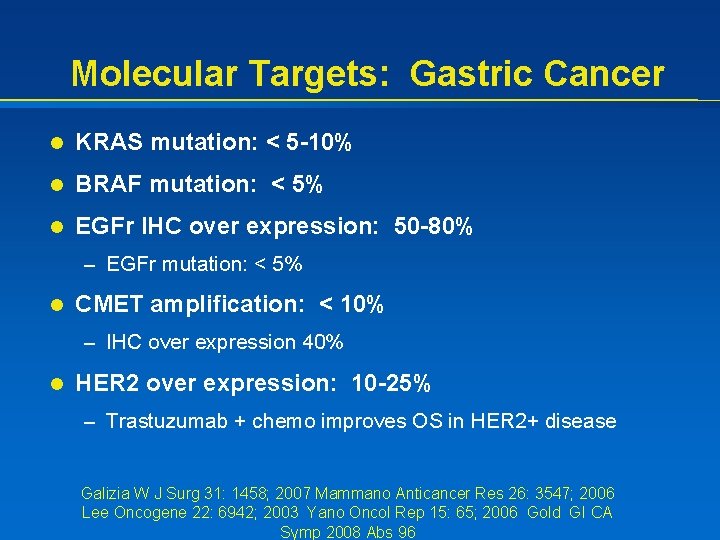
Molecular Targets: Gastric Cancer l KRAS mutation: < 5 -10% l BRAF mutation: < 5% l EGFr IHC over expression: 50 -80% – EGFr mutation: < 5% l CMET amplification: < 10% – IHC over expression 40% l HER 2 over expression: 10 -25% – Trastuzumab + chemo improves OS in HER 2+ disease Galizia W J Surg 31: 1458; 2007 Mammano Anticancer Res 26: 3547; 2006 Lee Oncogene 22: 6942; 2003 Yano Oncol Rep 15: 65; 2006 Gold GI CA Symp 2008 Abs 96

Targeted Agents Phase III: Met Disease l REAL 3: ECX + / - Panitumumab (U. K. ) l EXPAND: Cape-Cis + / Cetuximab l LOGIC: Cape-Ox + / - Lapatinib (HER 2+) l TYTAN: second line, paclitaxel + / Lapatinib (HER 2+) l Paclitaxel + / - Everolimus – GRANITE: Single agent Everolimus inactive, no improvement in OS, 656 patients

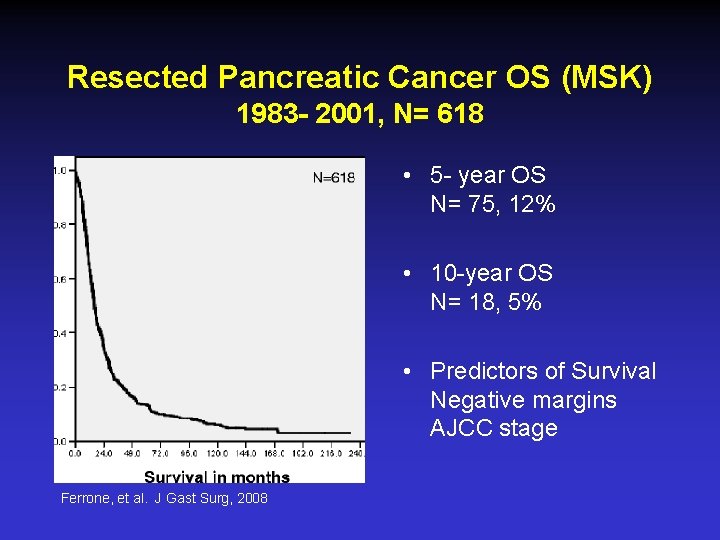
Resected Pancreatic Cancer OS (MSK) 1983 - 2001, N= 618 • 5 - year OS N= 75, 12% • 10 -year OS N= 18, 5% • Predictors of Survival Negative margins AJCC stage Ferrone, et al. J Gast Surg, 2008

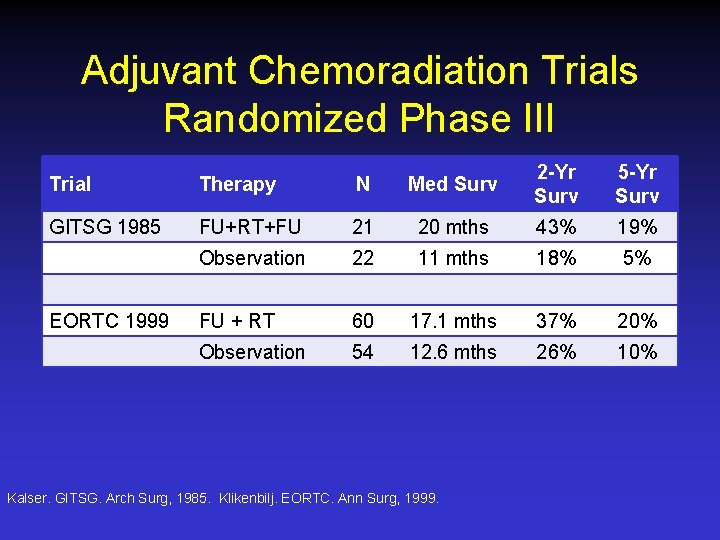
Adjuvant Chemoradiation Trials Randomized Phase III Trial Therapy N Med Surv 2 -Yr Surv 5 -Yr Surv GITSG 1985 FU+RT+FU 21 20 mths 43% 19% Observation 22 11 mths 18% 5% FU + RT 60 17. 1 mths 37% 20% Observation 54 12. 6 mths 26% 10% EORTC 1999 Kalser. GITSG. Arch Surg, 1985. Klikenbilj. EORTC. Ann Surg, 1999.

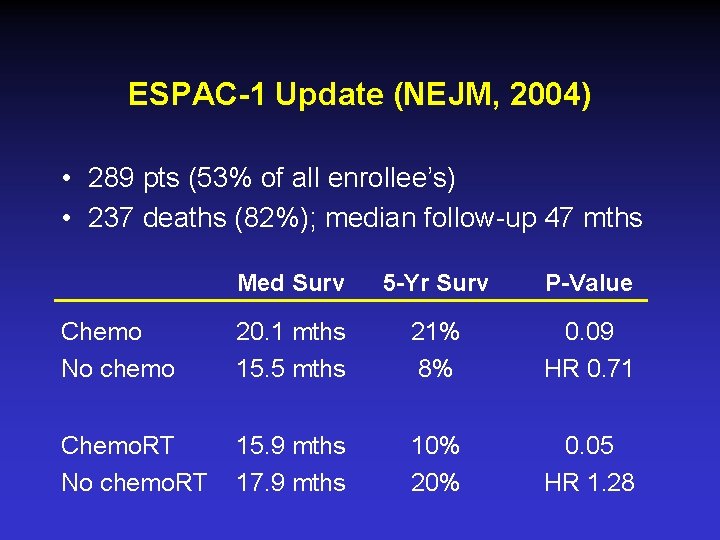
ESPAC-1 Update (NEJM, 2004) • 289 pts (53% of all enrollee’s) • 237 deaths (82%); median follow-up 47 mths Med Surv 5 -Yr Surv P-Value Chemo No chemo 20. 1 mths 15. 5 mths 21% 8% 0. 09 HR 0. 71 Chemo. RT No chemo. RT 15. 9 mths 17. 9 mths 10% 20% 0. 05 HR 1. 28

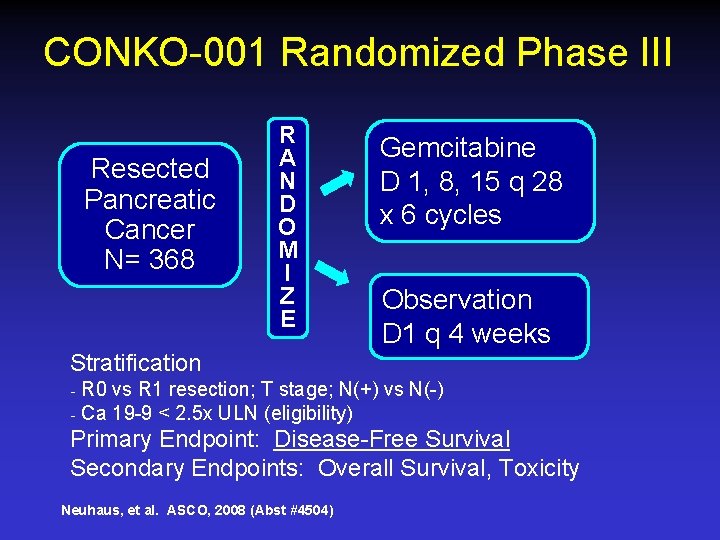
CONKO-001 Randomized Phase III Resected Pancreatic Cancer N= 368 R A N D O M I Z E Gemcitabine D 1, 8, 15 q 28 x 6 cycles Observation D 1 q 4 weeks Stratification ₋ R 0 vs R 1 resection; T stage; N(+) vs N(-) ₋ Ca 19 -9 < 2. 5 x ULN (eligibility) Primary Endpoint: Disease-Free Survival Secondary Endpoints: Overall Survival, Toxicity Neuhaus, et al. ASCO, 2008 (Abst #4504)

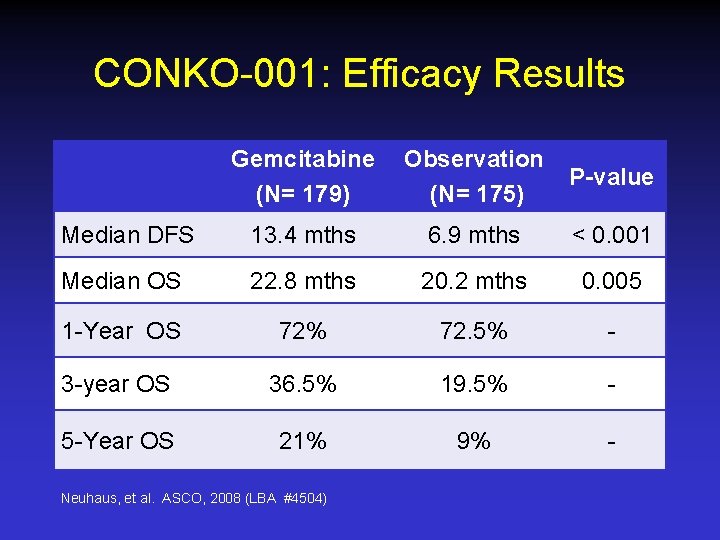
CONKO-001: Efficacy Results Gemcitabine (N= 179) Observation (N= 175) P-value Median DFS 13. 4 mths 6. 9 mths < 0. 001 Median OS 22. 8 mths 20. 2 mths 0. 005 1 -Year OS 72% 72. 5% - 3 -year OS 36. 5% 19. 5% - 5 -Year OS 21% 9% - Neuhaus, et al. ASCO, 2008 (LBA #4504)

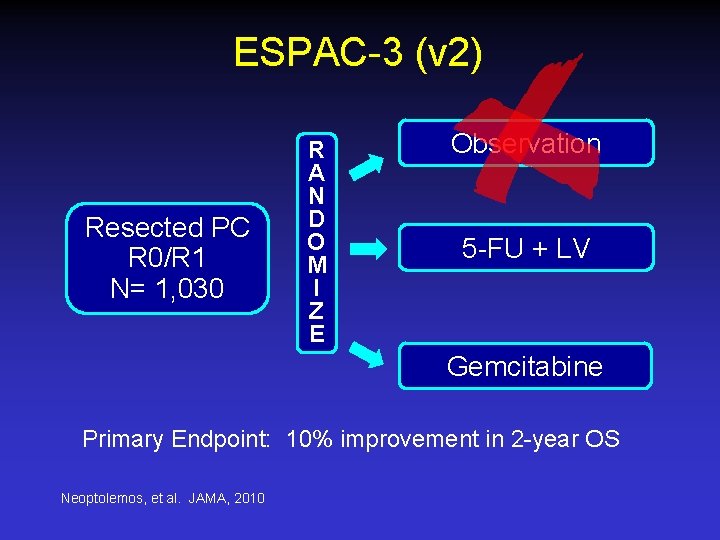
ESPAC-3 (v 2) Resected PC R 0/R 1 N= 1, 030 R A N D O M I Z E Observation 5 -FU + LV Gemcitabine Primary Endpoint: 10% improvement in 2 -year OS Neoptolemos, et al. JAMA, 2010

ESPAC-3 Overall Survival Median OS= 23 months Median OS= 23. 6 months c 2 LR=0. 74, p=0. 39, HRGEM VS 5 FU/FA=0. 94 (95%CI: 0. 81, 1. 08) LCTU Liverpool Cancer Trials Unit

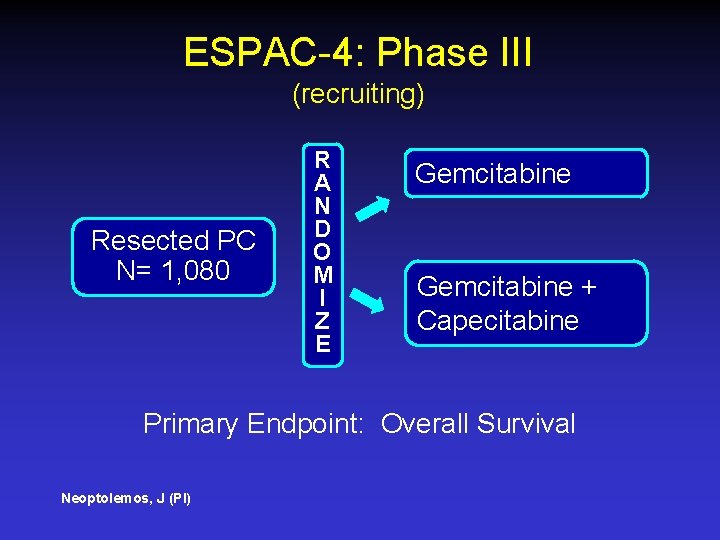
ESPAC-4: Phase III (recruiting) Resected PC N= 1, 080 R A N D O M I Z E Gemcitabine + Capecitabine Primary Endpoint: Overall Survival Neoptolemos, J (PI)

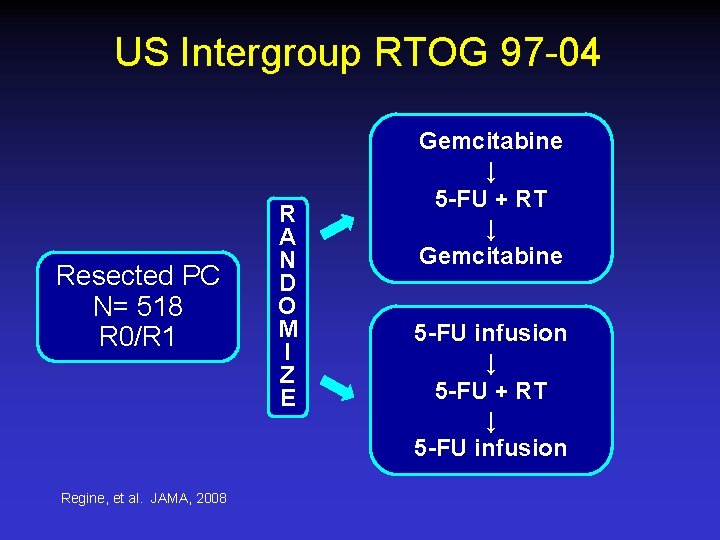
US Intergroup RTOG 97 -04 Resected PC N= 518 R 0/R 1 Regine, et al. JAMA, 2008 R A N D O M I Z E Gemcitabine ↓ 5 -FU + RT ↓ Gemcitabine 5 -FU infusion ↓ 5 -FU + RT ↓ 5 -FU infusion

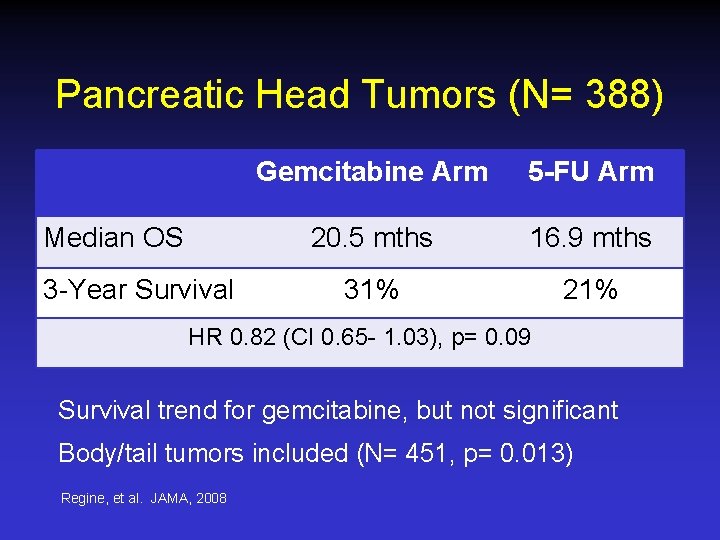
Pancreatic Head Tumors (N= 388) Median OS 3 -Year Survival Gemcitabine Arm 5 -FU Arm 20. 5 mths 16. 9 mths 31% 21% HR 0. 82 (CI 0. 65 - 1. 03), p= 0. 09 Survival trend for gemcitabine, but not significant Body/tail tumors included (N= 451, p= 0. 013) Regine, et al. JAMA, 2008

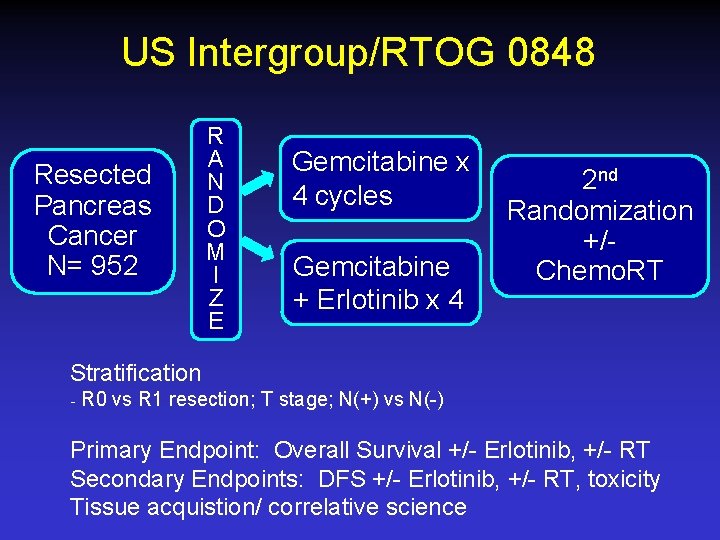
US Intergroup/RTOG 0848 Resected Pancreas Cancer N= 952 R A N D O M I Z E Gemcitabine x 4 cycles Gemcitabine + Erlotinib x 4 2 nd Randomization +/Chemo. RT Stratification ₋ R 0 vs R 1 resection; T stage; N(+) vs N(-) Primary Endpoint: Overall Survival +/- Erlotinib, +/- RT Secondary Endpoints: DFS +/- Erlotinib, +/- RT, toxicity Tissue acquistion/ correlative science

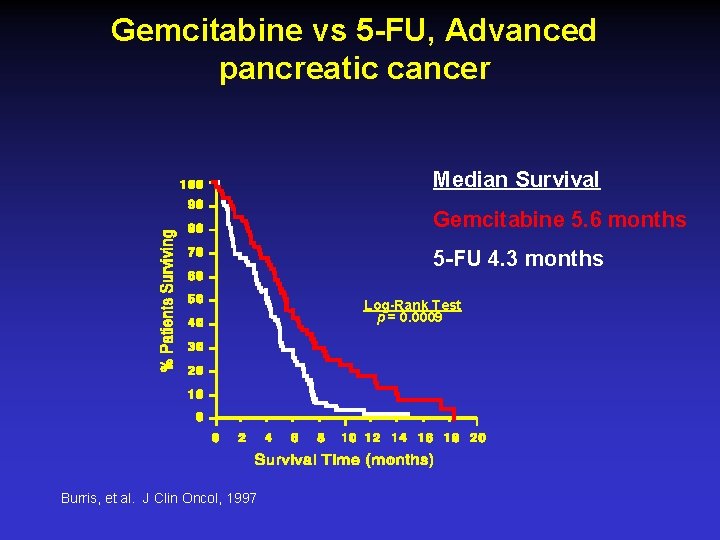
Gemcitabine vs 5 -FU, Advanced pancreatic cancer Median Survival Gemcitabine 5. 6 months 5 -FU 4. 3 months Log-Rank Test p = 0. 0009 Burris, et al. J Clin Oncol, 1997

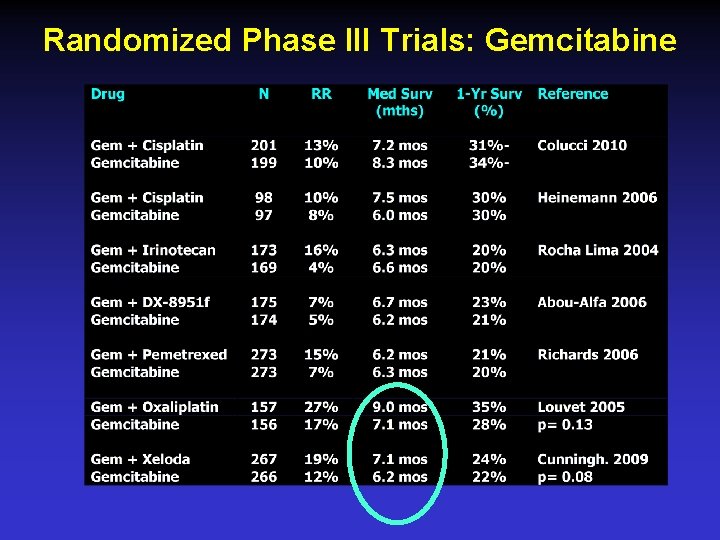
Randomized Phase III Trials: Gemcitabine

Gemcitabine vs Gemcitabine + Another Drug? Heinemann, BMC Cancer 8: 82; 2008: Meta Analysis HR Survival P-Value N Gem + platinum 0. 85 0. 01 623, 5 trials Gem + 5 -FU 0. 90 0. 03 901, 6 trials Good PS 90%+ Poor PS 60 - 80% 0. 76 1. 08 <0. 0001 1, 108, 5 trials 0. 40 574 Gemcitabine combination therapy: 10 -15% OS improvement

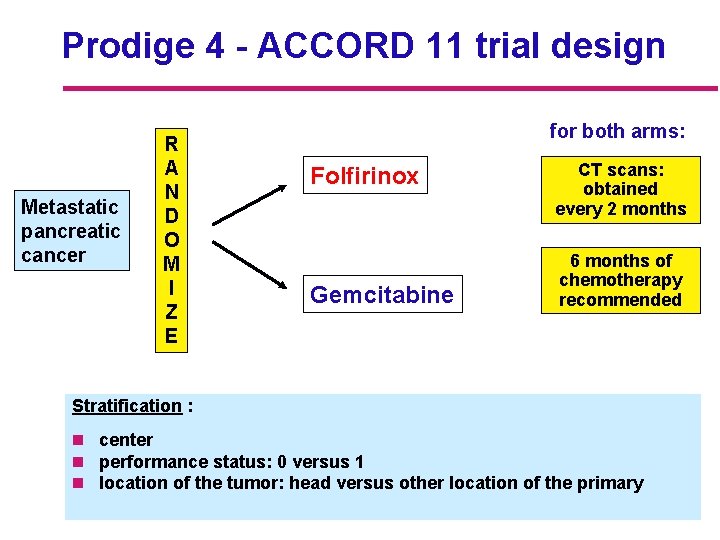
Prodige 4 - ACCORD 11 trial design Metastatic pancreatic cancer R A N D O M I Z E for both arms: Folfirinox Gemcitabine CT scans: obtained every 2 months 6 months of chemotherapy recommended Stratification : n center n performance status: 0 versus 1 n location of the tumor: head versus other location of the primary

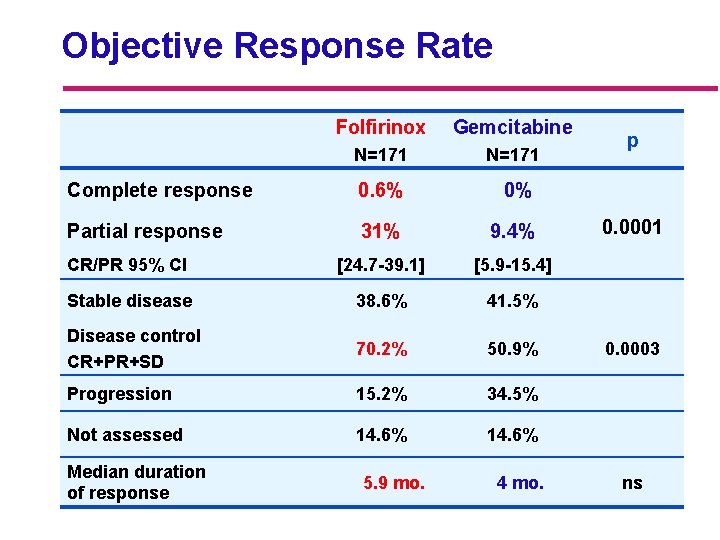
Objective Response Rate Folfirinox Gemcitabine N=171 Complete response 0. 6% 0% Partial response 31% 9. 4% CR/PR 95% CI [24. 7 -39. 1] [5. 9 -15. 4] Stable disease 38. 6% 41. 5% Disease control CR+PR+SD 70. 2% 50. 9% Progression 15. 2% 34. 5% Not assessed 14. 6% Median duration of response 5. 9 mo. 4 mo. p 0. 0001 0. 0003 ns

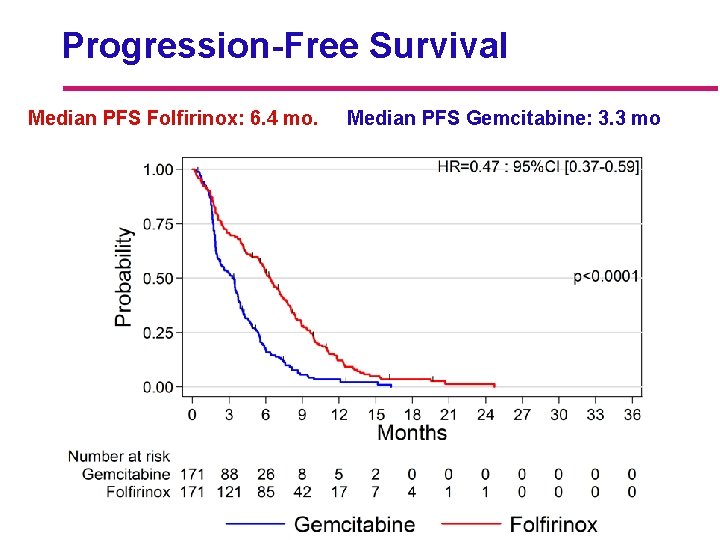
Progression-Free Survival Median PFS Folfirinox: 6. 4 mo. Median PFS Gemcitabine: 3. 3 mo

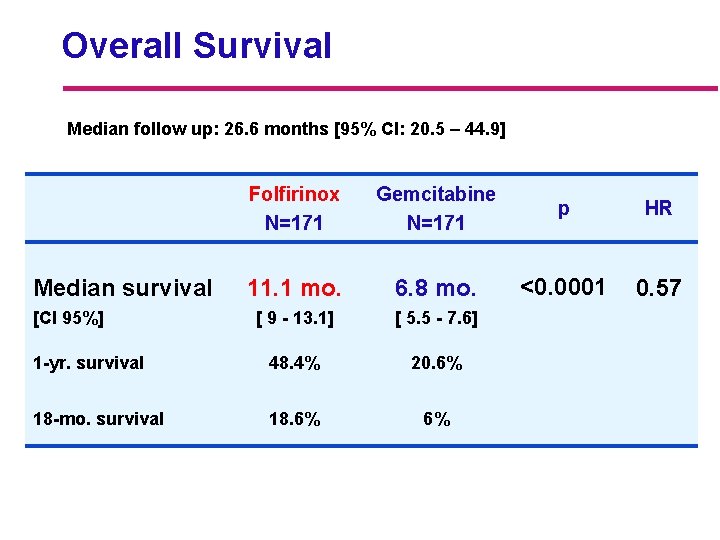
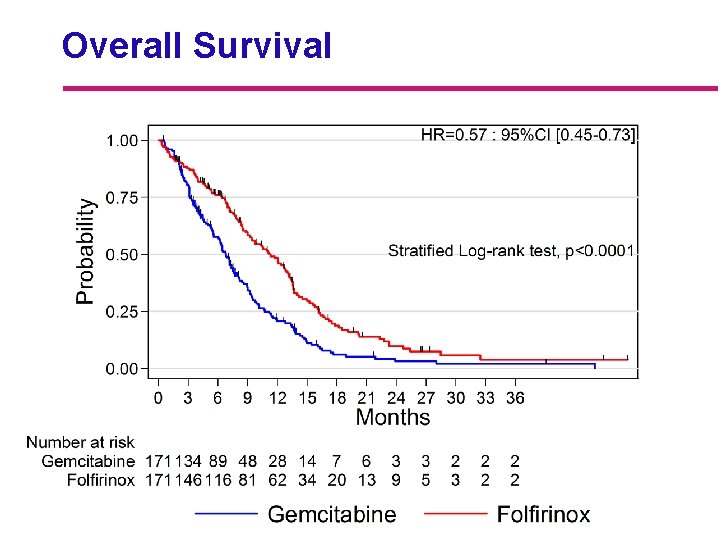
Overall Survival Median follow up: 26. 6 months [95% CI: 20. 5 – 44. 9] Folfirinox N=171 Gemcitabine N=171 p HR 11. 1 mo. 6. 8 mo. <0. 0001 0. 57 [ 9 - 13. 1] [ 5. 5 - 7. 6] 1 -yr. survival 48. 4% 20. 6% 18 -mo. survival 18. 6% 6% Median survival [CI 95%]

Overall Survival

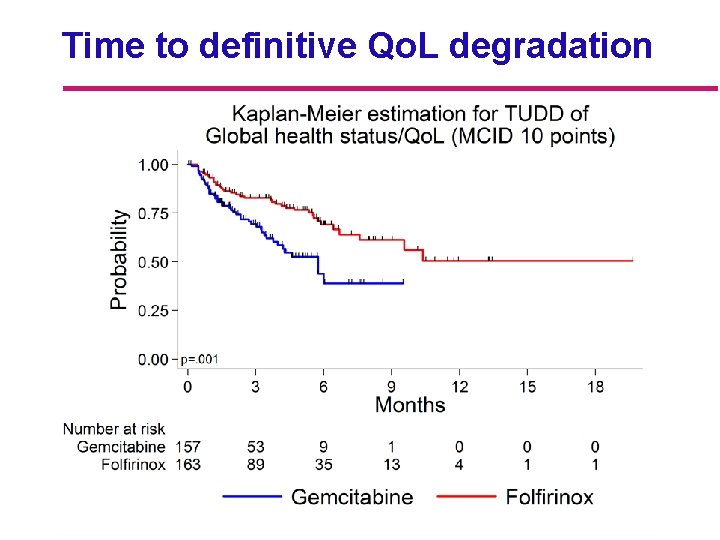
Time to definitive Qo. L degradation

Pancreatic Cancer • Chemotherapy with Gemcitabine has modest improvement in OS and QOL • Good PS patients may benefit for Gem + platin or Gem + 5 -FU • FOLFIRINOX is the new standard for good PS patients • Targeted Agents – Marginal benefit for Erlotinib – Negative results for Bevacizumab and Cetuximab

Pancreatic Cancer • Adjuvant chemotherapy with 5 -FU or Gemcitabine improves OS – Role of RT unclear – Current RTOG trial delivers RT at the end of chemo to select patients to best benefit – Locally unresectable disease • Similar approach of chemo first, selective use of RT if no POD