Updated August 2019 Protocol Updates Added 3122021 Protocol












- Slides: 23

Updated August 2019 Protocol Updates Added 3/12/2021

Protocol Updates Added A double blind placebo-controlled trial of Eflornithine and Sulindac to prevent recurrence of high risk adenomas and second primary colorectal cancers in patients with Stage 0 -III colon or rectal cancer, Phase III. 3/12/2021 S 0820

Powel H. Brown, M. D. , Ph. D. (Medical Oncology) M. D. Anderson Cancer Center Protocol Updates Added Jason A. Zell, D. O. , M. P. H. (Medical Oncology) UC Irvine Medical Center 3/12/2021 Study Coordinators

Protocol Updates Added Assess whether the combination of eflornithine and sulindac is effective in reducing the three-year event rate (high-risk adenomas and second primary colorectal cancers) in patients with previously treated Stage 0 -III colon or rectal cancer. 3/12/2021 Primary Objective

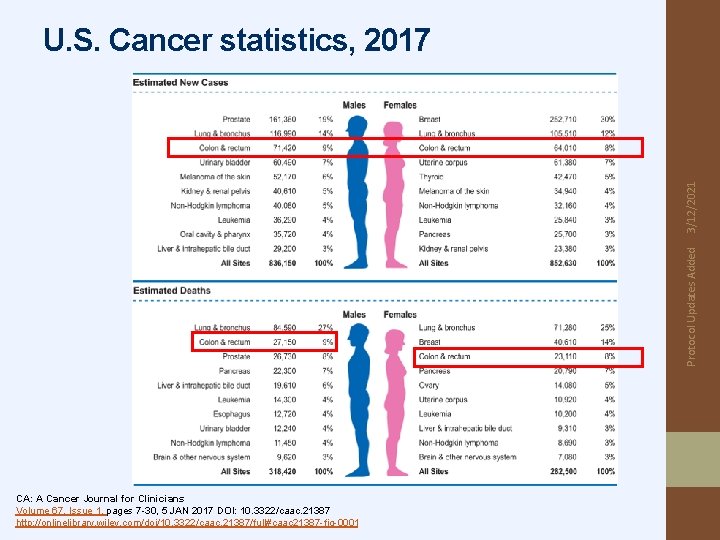
Protocol Updates Added 3/12/2021 U. S. Cancer statistics, 2017 CA: A Cancer Journal for Clinicians Volume 67, Issue 1, pages 7 -30, 5 JAN 2017 DOI: 10. 3322/caac. 21387 http: //onlinelibrary. wiley. com/doi/10. 3322/caac. 21387/full#caac 21387 -fig-0001

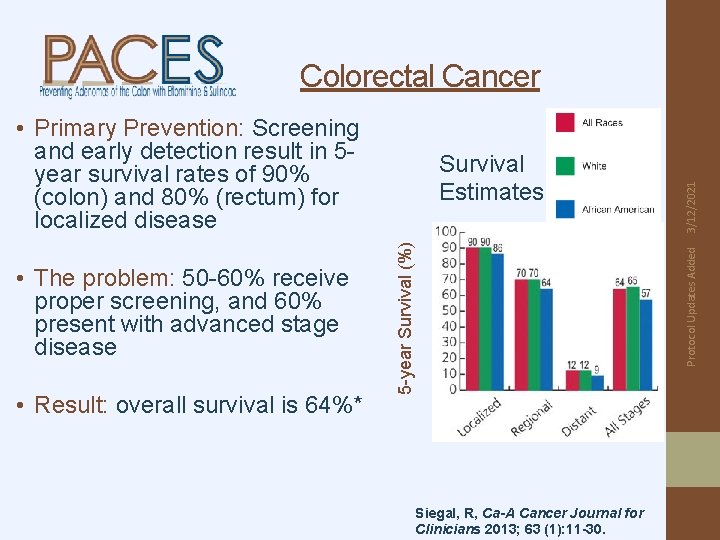
• Primary Prevention: Screening and early detection result in 5 year survival rates of 90% (colon) and 80% (rectum) for localized disease • Result: overall survival is 64%* Protocol Updates Added 5 -year Survival (%) • The problem: 50 -60% receive proper screening, and 60% present with advanced stage disease Survival Estimates 3/12/2021 Colorectal Cancer Siegal, R, Ca-A Cancer Journal for Clinicians 2013; 63 (1): 11 -30.

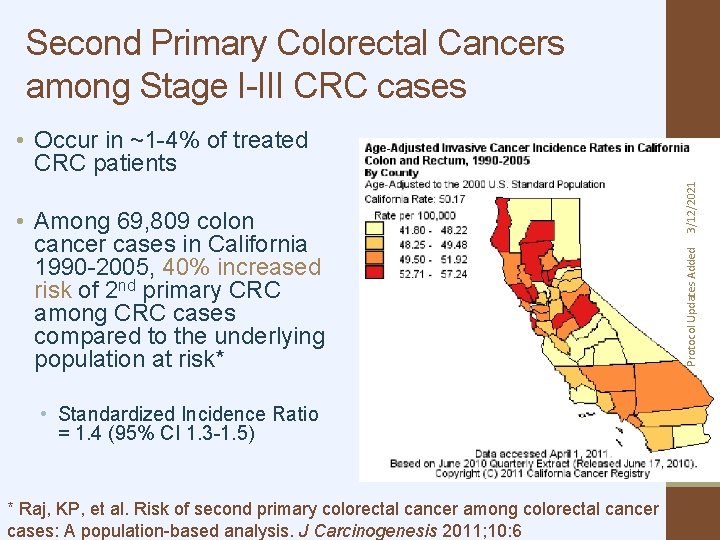
Second Primary Colorectal Cancers among Stage I-III CRC cases • Standardized Incidence Ratio = 1. 4 (95% CI 1. 3 -1. 5) * Raj, KP, et al. Risk of second primary colorectal cancer among colorectal cancer cases: A population-based analysis. J Carcinogenesis 2011; 10: 6 Protocol Updates Added • Among 69, 809 colon cancer cases in California 1990 -2005, 40% increased risk of 2 nd primary CRC among CRC cases compared to the underlying population at risk* 3/12/2021 • Occur in ~1 -4% of treated CRC patients

Protocol Updates Added 3/12/2021

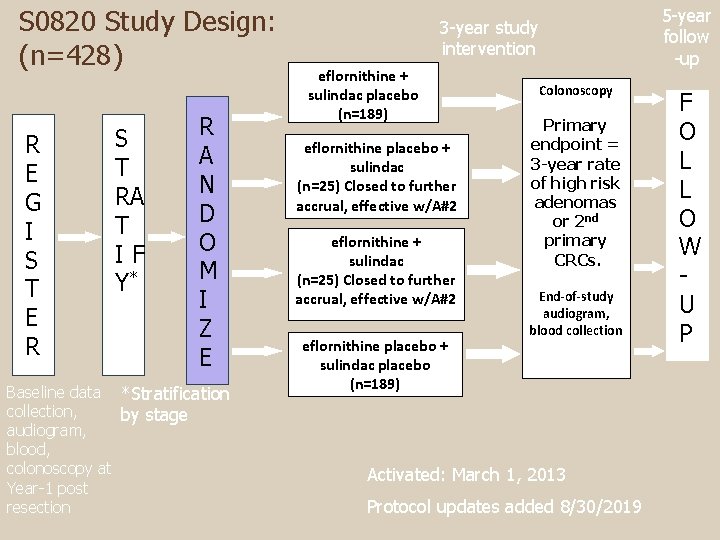
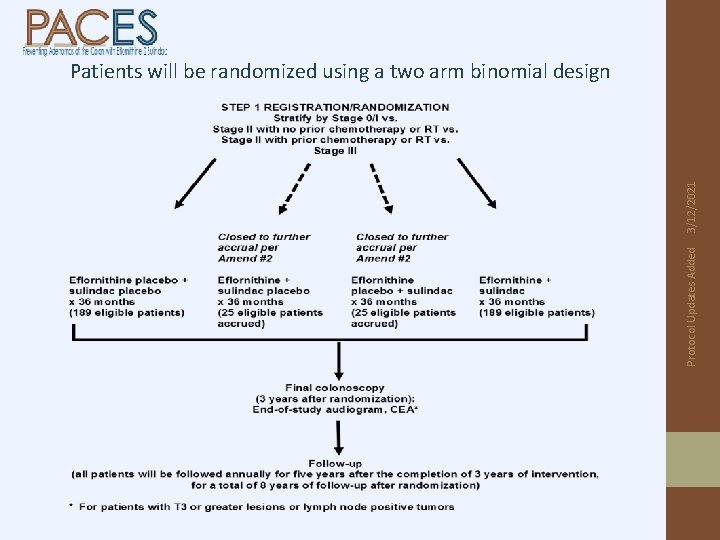
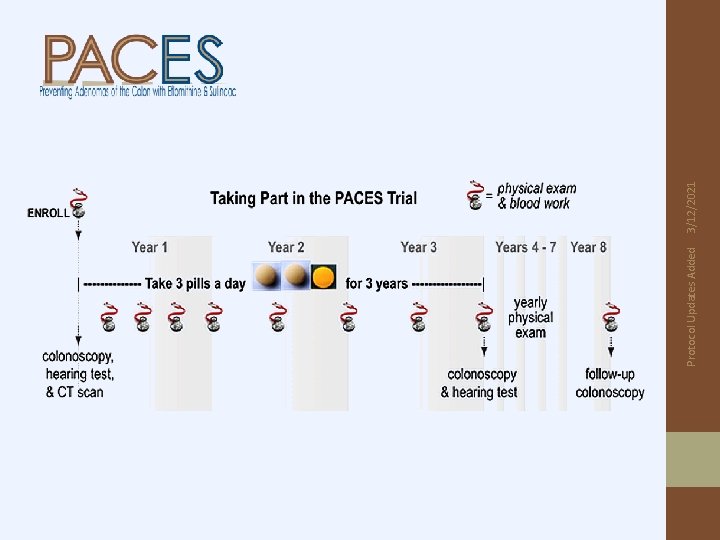
S 0820 Study Design: (n=428) R E G I S T E R S T RA T IF Y* R A N D O M I Z E Baseline data *Stratification collection, by stage audiogram, blood, colonoscopy at Year-1 post resection 5 -year follow -up 3 -year study intervention eflornithine + sulindac placebo (n=189) eflornithine placebo + sulindac (n=25) Closed to further accrual, effective w/A#2 eflornithine + sulindac (n=25) Closed to further accrual, effective w/A#2 eflornithine placebo + sulindac placebo (n=189) Colonoscopy Primary endpoint = 3 -year rate of high risk adenomas or 2 nd primary CRCs. End-of-study audiogram, blood collection Activated: March 1, 2013 Protocol updates added 8/30/2019 F O L L O W U P

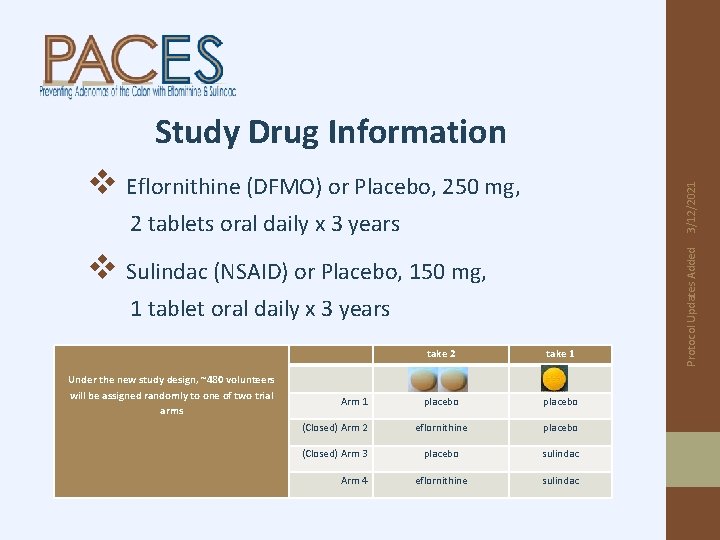
Study Drug Information 2 tablets oral daily x 3 years v Sulindac (NSAID) or Placebo, 150 mg, 1 tablet oral daily x 3 years take 2 take 1 placebo (Closed) Arm 2 eflornithine placebo (Closed) Arm 3 placebo sulindac eflornithine sulindac Under the new study design, ~480 volunteers will be assigned randomly to one of two trial arms Arm 1 Arm 4 Protocol Updates Added 3/12/2021 v Eflornithine (DFMO) or Placebo, 250 mg,

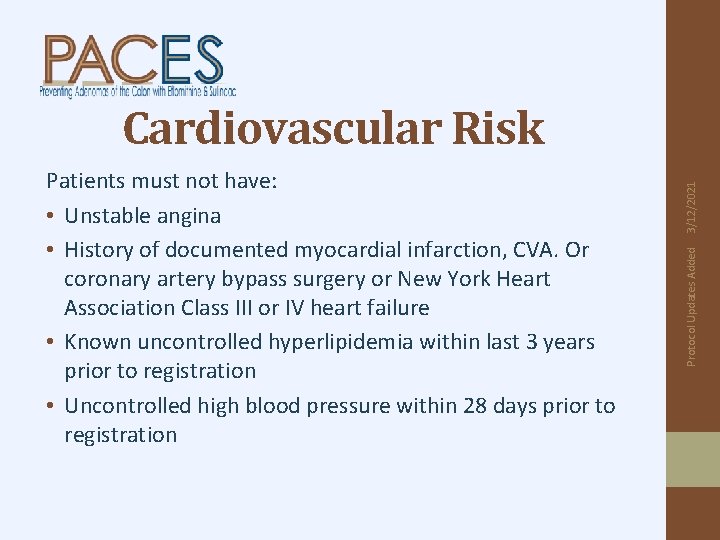
Protocol Updates Added Patients must not have: • Unstable angina • History of documented myocardial infarction, CVA. Or coronary artery bypass surgery or New York Heart Association Class III or IV heart failure • Known uncontrolled hyperlipidemia within last 3 years prior to registration • Uncontrolled high blood pressure within 28 days prior to registration 3/12/2021 Cardiovascular Risk

Protocol Updates Added • Stage 0, I, II or III colon or rectal cancer treated per standard care with resection alone or in combination with adjuvant chemotherapy • Adjuvant chemotherapy must have been completed at least 30 days prior to registration. • Patients with rectosigmoid cancers are eligible only if their treatment did NOT involve radiation therapy (i. e. neoadjuvant or adjuvant radiation) (mid-low rectal cancers are not eligible) • Had a primary resection one year previously and had a oneyear post-op colonoscopy showing no evidence of colorectal cancer. 3/12/2021 Eligibility

Protocol Updates Added • Must be at least 18 years of age • No history of familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, or inflammatory bowel disease, biallelic mismatch repair deficiency syndrome (BMMRD), or constitutional mismatch repair deficiency syndrome (CMMRD). • Must have a pure tone audiometry evaluation to document air conduction • Not be expecting to receive radiation or additional chemotherapy • Not be receiving or plan to receive concomitant corticosteroids NSAIDs, nor anticoagulants on a regular or predictable intermittent basis • Patients must have adequate blood counts 3/12/2021 Eligibility (continued)

Protocol Updates Added 3/12/2021 Patients will be randomized using a two arm binomial design

Year 1: H & P, CBC & chemistries every 3 months Year 2 & 3: H & P, CBC & chemistries every 6 months Colonoscopy & audiogram 3 years after randomization Patients followed annually for 5 years (after completion of 3 years of intervention): • H & P • Weight and Performance Status • Follow-up colonoscopy at 8 years post-registration per NCCN guidelines Protocol Updates Added • • 3/12/2021 Follow-up

Protocol Updates Added 3/12/2021

• • Communicate with patient to define barriers Review patient’s routine for taking study drugs Seek family assistance Consider more frequent contact with patient using phone check-in Protocol Updates Added • Study drug adherence measured by returned tablet count • Unused medication returned to the clinic at each visit • Patients document study drug intake on an Intake Calendar • Individualized adherence strategies used if adherence is ↓ 75%: 3/12/2021 Adherence

• Event = high risk adenoma or 2 nd primary colorectal cancer • High risk adenoma = either advanced adenoma (villous or tubulovillous histology, size ≥ 1 cm, or high grade dysplasia) or multiple adenomas (3 or more each > 0. 3 cm) Protocol Updates Added • 3 -year event rate after registration among Stage 0 -III colorectal cancer patients 3/12/2021 Primary Endpoint

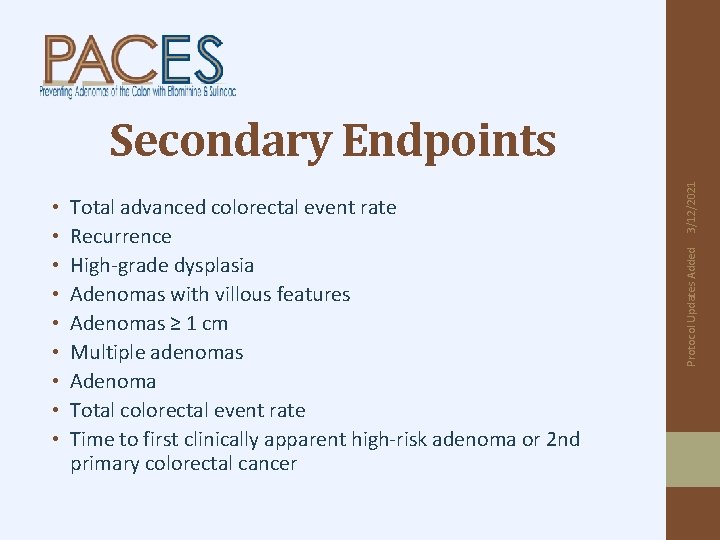
Total advanced colorectal event rate Recurrence High-grade dysplasia Adenomas with villous features Adenomas ≥ 1 cm Multiple adenomas Adenoma Total colorectal event rate Time to first clinically apparent high-risk adenoma or 2 nd primary colorectal cancer Protocol Updates Added • • • 3/12/2021 Secondary Endpoints

• including genotyping and biochemical assays • fasting whole blood sample required after registration and prior to beginning treatment • Diet and Lifestyle Substudy: • completion of Diet & Lifestyle Questionnaires after registration Protocol Updates Added • Submission of blood specimen for nutritional assays and banking: 3/12/2021 Optional for patients…….

Timing: ü Patients must be registered between 120 days and 456 days (inclusive) of primary colon or rectal resection ü Colonoscopy performed at least 120 days after resection date and prior to registration must show no evidence of colorectal cancer ü Polyps detected at colonoscopy must be completely removed ü Adjuvant chemo and RT treatment completed ≥ 30 days prior to registration Protocol Updates Added üHistory of stage 0, I, II or III colon or rectal adenocarcinoma üTreated with resection alone or in combination with adjuvant chemotherapy üNED at one year post-operative assessment 3/12/2021 Please help identify potential patients

Materials for the Investigators and Staff include: • Notification of Registration letter • FAQs • Adherence Strategies • Protocol Card Materials for the patient include: • Thank You Letter from the PI • List of Resources & Advocates Contacts • Printable birthday cards Protocol Updates Added Recruitment Materials available: • Professional Slide Set • Patient Brochure • Promotional Letter to Physicians • Promotional Flyer 3/12/2021 S 0820 Web Page

Thank You Protocol Updates Added 3/12/2021 S 0820