Update to IPC on MODEL QUALITY ASSURANCE SYSTEM



















- Slides: 24

Update to IPC on MODEL QUALITY ASSURANCE SYSTEM FOR PROCUREMENT AGENCIES (MQAS) Dr Sabine Kopp 16 December 2014

MODEL QUALITY ASSURANCE SYSTEM FOR PROCUREMENT AGENCIES (MQAS) l WHO Expert Committee on Specifications for Pharmaceutical Preparations of the World Health Organization (WHO) adopted in 2005 (Ref: Annex 6 in the Technical Report Series, No. 937 in 2006) l Procurement organizations have implemented the recommendations presented in the MQAS l Donor organizations (including the Global Fund to Fight AIDs, Tuberculosis and Malaria (GFATM)) have endorsed the MQAS as part of their quality assurance policy for the procurement of pharmaceutical products with their funds

MQAS Subsequently adopted as Interagency guideline by: l UNICEF l UNDP l UNFPA l World Bank l WHO

New developments since August 2011 l Based on implementation -> Identification of need for revision and the need for an Assessment Tool for procurement agencies during meeting organized by WHO/The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) l Two informal meetings held by the Global Fund to prepare the new Assessment Tool and to review the MQAS; active Working Group created to develop a harmonized Assessment Tool

Working group members Representatives from: - Committee for Medicinal Products for Human Use (CHMP), - Crown Agents, - Global Drug Facility (GDF), - International Committee of the Red Cross (ICRC), International Development Association (IDA), - Médecins Sans Frontières (MSF), - Management Sciences for Health (MSH), - Partnership for Supply Chain Management (PFSCM), - Quality Medicines for All (QUAMED), International Union Against Tuberculosis and Lung Disease (The Union), - United Nations Children’s Fund (UNICEF), United Nations Office for Project Services (UNOPS), - United States Agency for International Development (USAID) and WHO

Progress during 2012 and 2013 l Endorsement of revision process by 47 th WHO Expert Committee on Specifications for Pharmaceutical Preparations l Review of comments received during circulation and during the Expert Committee meeting, in meeting of the Working Group (WG), preparation of revised MQAS l Informal consultation coorganized by GFATM and WHO’s Quality Assurance Programme: MQAS revision and aidememoire, model inspection report and self-inspection tool reviewed, newly revised working documents prepared based on discussion and feedback by GFATM and WG

Revision Process + The revision process of MQAS has resulted in the following : l newly proposed revised text of the MQAS (QAS/12. 508/Rev. 1); l a revised Product Questionnaire (QAS/13. 556); l an Assessment Tool (QAS/13. 558), together with l an Inspection report format (QAS/13. 557); l an aide-memoire for the inspection (QAS/13. 555).

Next steps l Public consultation: http: //www. who. int/medicines/areas/quality_safety/quality_ assurance/projects/en/index. html l Presented to 48 th WHO Expert Committee on Specifications for Pharmaceutical Preparations (ECSPP) for possible adoption (14 -18 October 2013) 4 new texts adopted by ECSPP "Ready for use"

WHO Governing bodies (EB)… 48 th WHO Expert Committee on Specifications for Pharmaceutical Preparations (TRS 986) – in May 2014

WHO TRS 986 l Annex 3 - Model quality assurance system for procurement agencies, including 2 new appendices: – a revised model inspection report and – a revised interagency product questionnaire l Annex 4: Assessment tool based on the model quality assurance system for procurement agencies: aidememoire for inspection l French translation in preparation for e-publication!

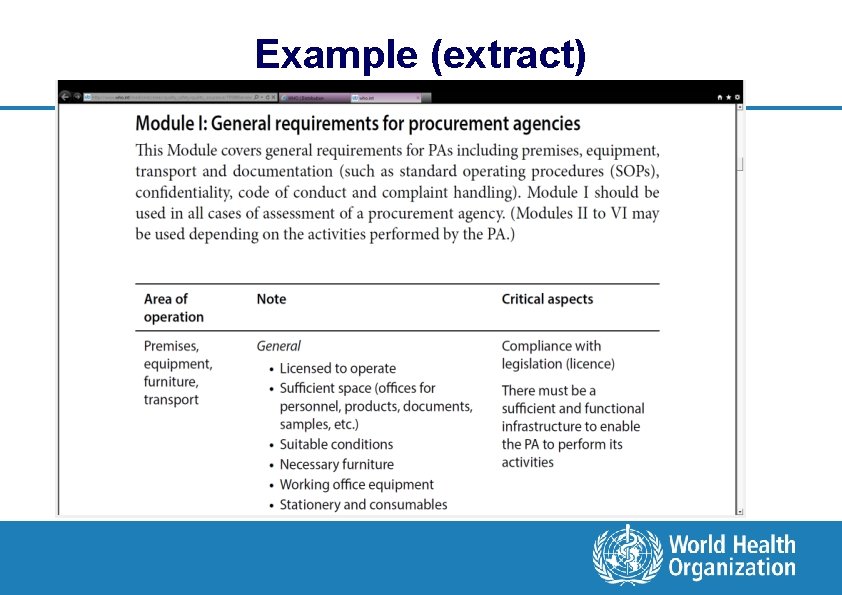
MQAS what does it cover? l Module I: General requirements for procurement agencies l Module II: Prequalification l Module III: Purchasing l Module IV: Receipt and storage of purchased products l Module V: Distribution l Module VI: Reassessment l Annexes

MQAS: a comprehensive publication l Annex 1. Example of a code of conduct l Annex 2. Example of a guideline on confidentiality l Annex 3. Example of a guideline on conflict of interest l Annex 4. Example of a standard operating procedure (SOP) for writing an SOP l Annex 5. Example of an invitation for expression of interest l Annex 6. Interagency finished pharmaceutical product questionnaire (revised as "interagency")

l Annex 7. Example of a standard operating procedure for screening and assessing product information l Annex 8. Quality systems recommendations for pharmaceutical inspectorates l Annex 9: Technical questionnaire for pharmaceutical manufacturers l Annex 10. Example of a standard operating procedure for planning of inspections

l Annex 11. Example of a standard operating procedure for preparing for an inspection l Annex 12. Example of a standard operating procedure for performing an inspection l Annex 13. Example of a checklist for good manufacturing practices l Annex 14. Guidance on good manufacturing practices: inspection report (revised) l Annex 15. Good storage practice l Annex 16. Good trade and distribution practices (update in pipeline)

Annex 4: Assessment tool : aide-memoire for inspection Purpose l Harmonized tool to result in better use of resources by coordinating procurement agency (PA) assessments towards mutual recognition of the findings Scope l Assessment tool is based on the six modules in the MQAS

Annex 4: Assessment tool : aide-memoire for inspection Assessment tool l Should be used by qualified, experienced persons when assessing a PA (including wholesalers and distributors) for compliance with recommended international standards l Can also be useful for a PA in self-assessment l Is not a checklist, but serves as a document to help and remind inspectors as to what should be assessed during inspections of PAs

Example (extract)

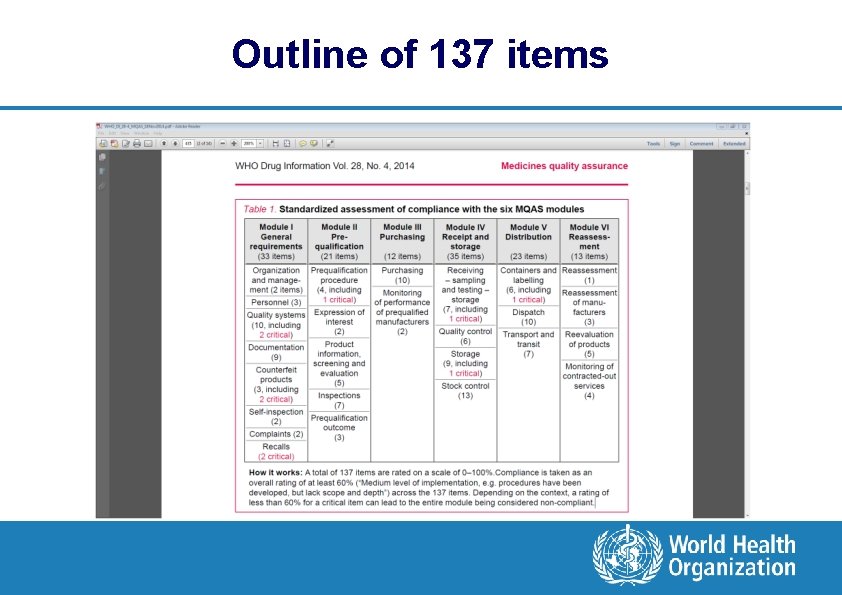
A harmonized self-assessment tool for procurement agencies l Working group developed also a harmonized compliance self -assessment tool based on the six MQAS modules l Evaluation includes % ratings allocated to 137 items l Tool supplements the formal WHO guidance texts by providing a consistent, flexible way to measure implementation of principles defined in MQAS l Tool enables to communicate outcomes in a standardized way, and to take targeted measures for improvement l Ref: WHO Drug Information Vol. 28, No. 4, 2014, p. 434 -447

Outline of 137 items

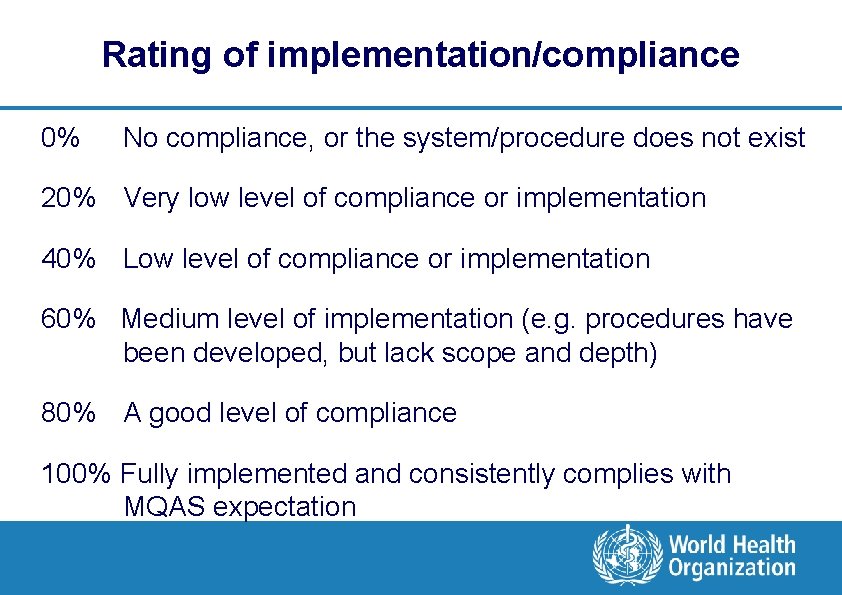
Rating of implementation/compliance 0% No compliance, or the system/procedure does not exist 20% Very low level of compliance or implementation 40% Low level of compliance or implementation 60% Medium level of implementation (e. g. procedures have been developed, but lack scope and depth) 80% A good level of compliance 100% Fully implemented and consistently complies with MQAS expectation

Example of items assessed

Model report format

Future steps New MQAS and assessment tools are "Ready for use" by procurement agencies and national/regional authorities IPC/Agencies may envisage updating the interagency guidelines publication

http: //www. who. int/medicines/areas/quality_safety/quality_assurance/distribution/en / Safe quality medicines