Update on the BIOSOLVE II and III Clinical

Update on the BIOSOLVE II and III Clinical Trials Using MAGMARIS Absorbable Magnesium Scaffold Michael Haude, MD, Ph. D Städtische Kliniken Neuss Lukaskrankenhaus Gmb. H Neuss, Germany

Disclosures: Speaker's name: Michael Haude Grant support: Biotronik, Orbus Neich, Abbott, Medtronic, Cardiac Dimensions Speaker´s bureau: Biotronik, Orbus Neich, Abbott, Medtronic, Lilly, Volcano, Cardiac Dimensions (Proctor) Consultant: Biotronik, Orbus Neich, Abbott
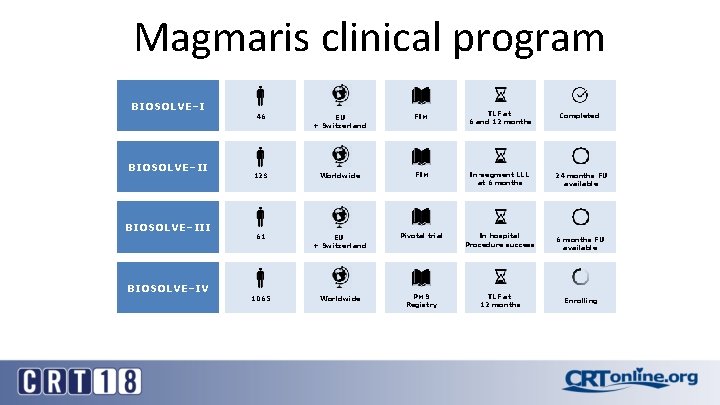
Magmaris clinical program BIOSOLVE–III BIOSOLVE–IV 46 EU + Switzerland FIM TLF at 6 and 12 months Completed 123 Worldwide FIM In-segment LLL at 6 months 24 months FU available 61 EU + Switzerland Pivotal trial In hospital Procedure success 1065 Worldwide PMS Registry TLF at 12 months 6 months FU available Enrolling
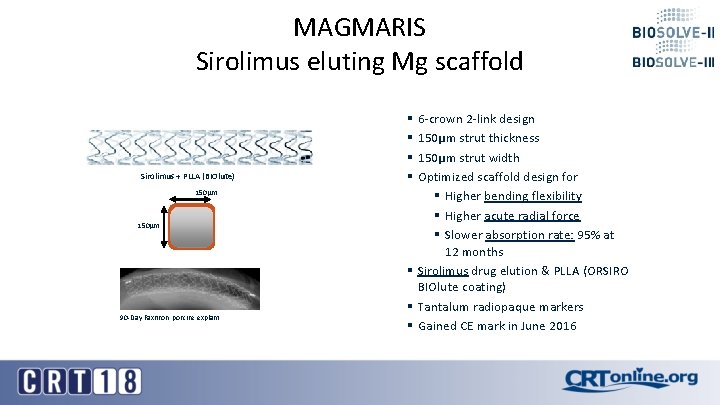
MAGMARIS Sirolimus eluting Mg scaffold Sirolimus + PLLA (BIOlute) 150µm 90 -Day Faxitron, porcine explant § 6 -crown 2 -link design § 150µm strut thickness § 150µm strut width § Optimized scaffold design for § Higher bending flexibility § Higher acute radial force § Slower absorption rate: 95% at 12 months § Sirolimus drug elution & PLLA (ORSIRO BIOlute coating) § Tantalum radiopaque markers § Gained CE mark in June 2016

BIOSOLVE-II 24 m follow-up BIOSOLVE-II/III combined 6 m follow-up
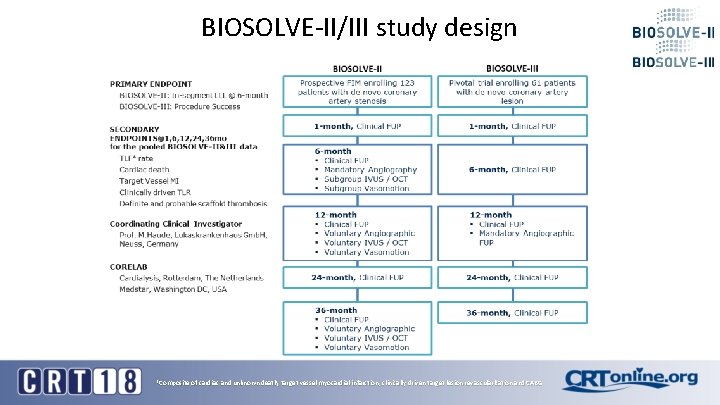
BIOSOLVE-II/III study design *Composite of cardiac and unknown death, target vessel myocardial infarction, clinically driven target lesion revascularization and CABG
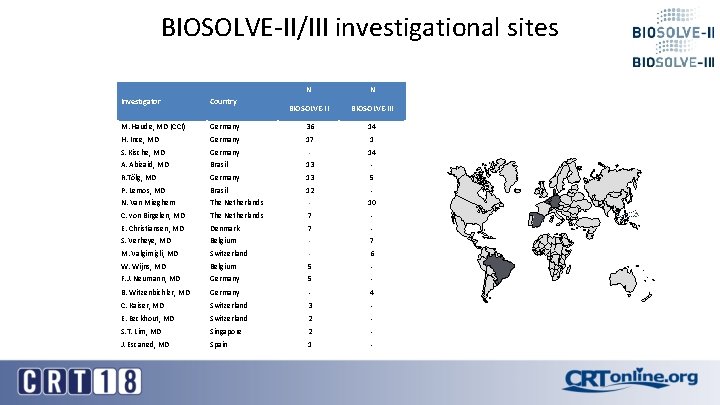
BIOSOLVE-II/III investigational sites N N BIOSOLVE-III 14 Investigator Country M. Haude, MD (CCI) Germany 36 H. Ince, MD Germany 17 1 S. Kische, MD Germany - 14 A. Abizaid, MD Brasil 13 - R. Tölg, MD Germany 13 5 P. Lemos, MD Brasil 12 - N. Van Mieghem The Netherlands - 10 C. von Birgelen, MD The Netherlands 7 - E. Christiansen, MD Denmark 7 - S. Verheye, MD Belgium - 7 M. Valgimigli, MD Switzerland - 6 W. Wijns, MD Belgium 5 - F. J. Neumann, MD Germany 5 - B. Witzenbichler, MD Germany - 4 C. Kaiser, MD Switzerland 3 - E. Eeckhout, MD Switzerland 2 - S. T. Lim, MD Singapore 2 - J. Escaned, MD Spain 1 -
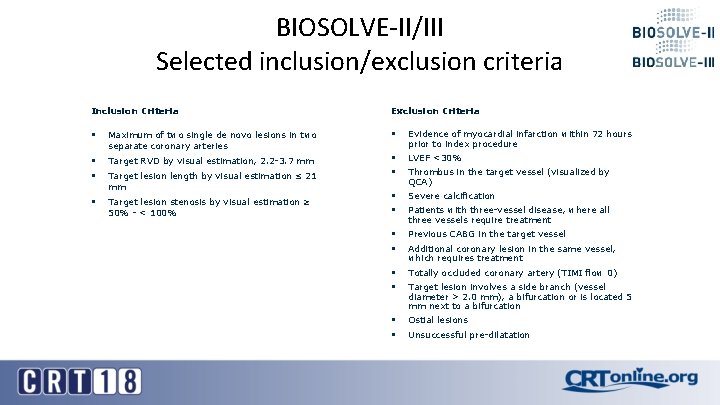
BIOSOLVE-II/III Selected inclusion/exclusion criteria Inclusion Criteria Exclusion Criteria § Maximum of two single de novo lesions in two separate coronary arteries § Evidence of myocardial infarction within 72 hours prior to index procedure § Target RVD by visual estimation, 2. 2 -3. 7 mm LVEF <30% § Target lesion length by visual estimation ≤ 21 mm § § § Target lesion stenosis by visual estimation ≥ 50% - < 100% § § Severe calcification § § Previous CABG in the target vessel § § Totally occluded coronary artery (TIMI flow 0) § § Ostial lesions Thrombus in the target vessel (visualized by QCA) Patients with three-vessel disease, where all three vessels require treatment Additional coronary lesion in the same vessel, which requires treatment Target lesion involves a side branch (vessel diameter > 2. 0 mm), a bifurcation or is located 5 mm next to a bifurcation Unsuccessful pre-dilatation
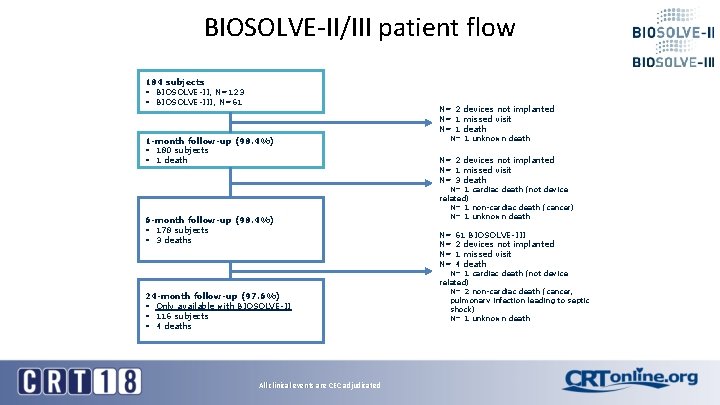
BIOSOLVE-II/III patient flow 184 subjects § BIOSOLVE-II, N=123 § BIOSOLVE-III, N=61 N= 2 devices not implanted N= 1 missed visit N= 1 death 1 -month follow-up (98. 4%) § 180 subjects § 1 death 6 -month follow-up (98. 4%) § 178 subjects § 3 deaths 24 -month follow-up (97. 6%) § Only available with BIOSOLVE-II § 116 subjects § 4 deaths All clinical events are CEC adjudicated N= 1 unknown death N= 2 devices not implanted N= 1 missed visit N= 3 death N= 1 cardiac death (not device related) N= 1 non-cardiac death (cancer) N= 1 unknown death N= N= 61 BIOSOLVE-III 2 devices not implanted 1 missed visit 4 death N= 1 cardiac death (not device related) N= 2 non-cardiac death (cancer, pulmonary infection leading to septic shock) N= 1 unknown death

BIOSOLVE-II/III Baseline and lesion characteristics Baseline Characteristics N=184 Lesion Location N=189 BIOSOLVE-II N (%) BIOSOLVE-III N (%) 65. 2 ± 10. 3 66. 3 ± 11. 8 LAD 47 (38. 2) 31 (47. 0) Male 78 (63. 4) 39 (63. 9) LCx 29 (23. 6) 15 (22. 7) Hypertension 101 (82. 1) 45 (73. 8) RCA 45 (36. 6) 19 (28. 8) Hyperlipidemia 74 (60. 2) 40 (65. 6) 2 (1. 6) 1 (1. 5) Smoking 67 (54. 5) 35 (57. 4) Diabetes mellitus 36 (29. 3) 10 (16. 4) -Insulin dependent 11 (30. 6) 1 (10. 0) -Non-Insulin dependent 25 (69. 4) 9 (90. 0) History of MI 29 (23. 6) 14 (23. 0) Previous percutaneous Intervention 52 (42. 3) 24 (39. 3) Age (mean ± SD) Intermediate Branch Lesion Characteristics N=189 BIOSOLVE-II N (%) BIOSOLVE-III N (%) Lesion Length (mm ± SD) 12. 6 ± 4. 5 12. 2 ± 6. 1 RVD (mm ± SD) 2. 7 ± 0. 40 2. 8 ± 0. 50 53 (43. 4) 53 (80. 3) 13 (10. 7) 16 (24. 2) AHA/ ACC Lesion Class B 2/C* Calcification Moderate/Severe* * Statistical Significant differences between BIOSOLVE-II and BIOSOLVE-III population
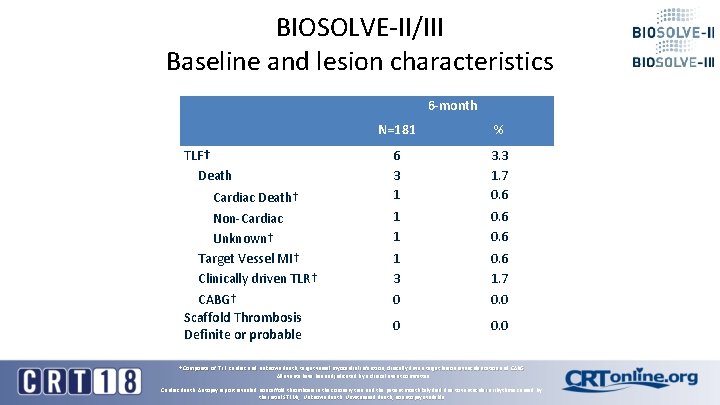
BIOSOLVE-II/III Baseline and lesion characteristics 6 -month TLF† Death Cardiac Death† Non-Cardiac Unknown† Target Vessel MI† Clinically driven TLR† CABG† Scaffold Thrombosis Definite or probable N=181 % 6 3 1 3. 3 1. 7 0. 6 1 1 0. 6 1 3 0 0. 6 1. 7 0. 0 0 0. 0 † Composite of TLF: cardiac and unknown death, target vessel myocardial infarction, clinically driven target lesion revascularization and CABG All events have been adjudicated by a clinical event committee Cardiac death: Autopsy report revealed no scaffold thrombosis in the coronary tree and the patient most likely died due to ventricular arrhythmia caused by the initial STEMI; Unknown death: Unwitnessed death, no autopsy available
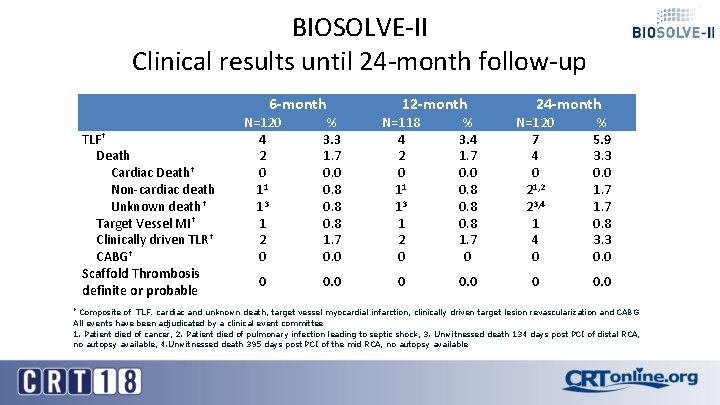
BIOSOLVE-II Clinical results until 24 -month follow-up TLF† Death Cardiac Death† Non-cardiac death Unknown death† Target Vessel MI† Clinically driven TLR† CABG† Scaffold Thrombosis definite or probable 6 -month 12 -month 24 -month N=120 4 2 0 11 13 1 2 0 % 3. 3 1. 7 0. 0 0. 8 1. 7 0. 0 N=118 4 2 0 11 13 1 2 0 % 3. 4 1. 7 0. 0 0. 8 1. 7 0 N=120 7 4 0 21, 2 23, 4 1 4 0 % 5. 9 3. 3 0. 0 1. 7 0. 8 3. 3 0. 0 0 0. 0 Composite of TLF: cardiac and unknown death, target vessel myocardial infarction, clinically driven target lesion revascularization and CABG All events have been adjudicated by a clinical event committee 1. Patient died of cancer, 2. Patient died of pulmonary infection leading to septic shock, 3. Unwitnessed death 134 days post PCI of distal RCA, no autopsy available, 4. Unwitnessed death 395 days post PCI of the mid RCA, no autopsy available †
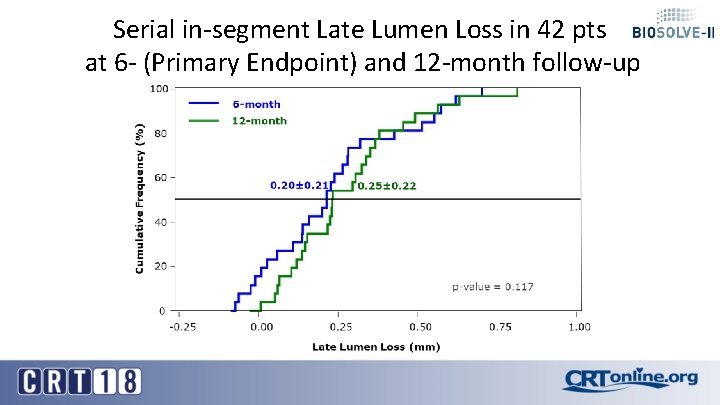
Serial in-segment Late Lumen Loss in 42 pts at 6 - (Primary Endpoint) and 12 -month follow-up
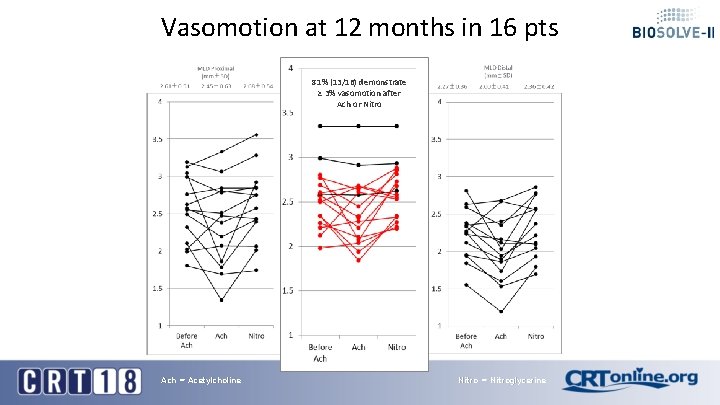
Vasomotion at 12 months in 16 pts 81% (13/16) demonstrate ≥ 3% vasomotion after Ach or Nitro Ach = Acetylcholine Nitro = Nitroglycerine
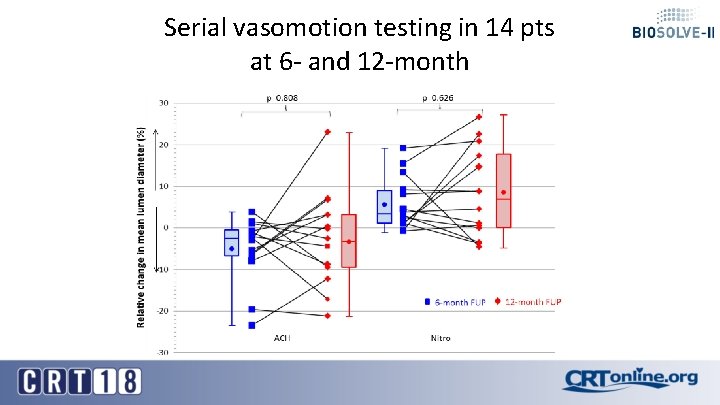
Serial vasomotion testing in 14 pts at 6 - and 12 -month
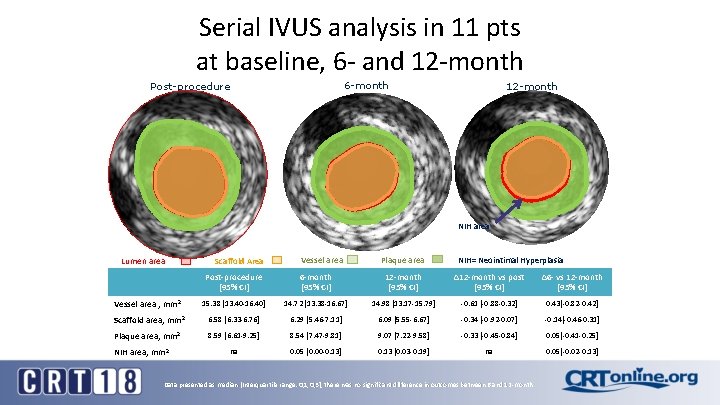
Serial IVUS analysis in 11 pts at baseline, 6 - and 12 -month 6 -month Post-procedure 12 -month NIH area Lumen area Scaffold Area Vessel area Plaque area NIH= Neointimal Hyperplasia Post-procedure [95% CI] 6 -month [95% CI] 12 -month [95% CI] ∆12 -month vs post [95% CI] ∆6 - vs 12 -month [95% CI] Vessel area , mm 2 15. 38 [13. 40 -16. 40] 14. 72 [13. 38 -16. 67] 14. 98 [13. 17 -15. 79] -0. 61 [-0. 88 -0. 32] 0. 43[-0. 82 -0. 42] Scaffold area, mm 2 6. 58 [6. 33 -6. 76] 6. 29 [5. 46 -7. 11] 6. 09 [5. 55 -6. 67] -0. 34 [-0. 92 -0. 07] -0. 14[-0. 46 -0. 31] Plaque area, mm 2 8. 59 [6. 61 -9. 25] 8. 54 [7. 47 -9. 81] 9. 07 [7. 22 -9. 58] -0. 33 [-0. 45 -0. 84] 0. 05[-0. 41 -0. 25] na 0. 05 [0. 00 -0. 13] 0. 13 [0. 03 -0. 19] na 0. 05[-0. 02 -0. 13] NIH area, mm 2 Data presented as median [Interquartile range: Q 1, Q 3], there was no significant difference in outcomes between 6 and 12 -month
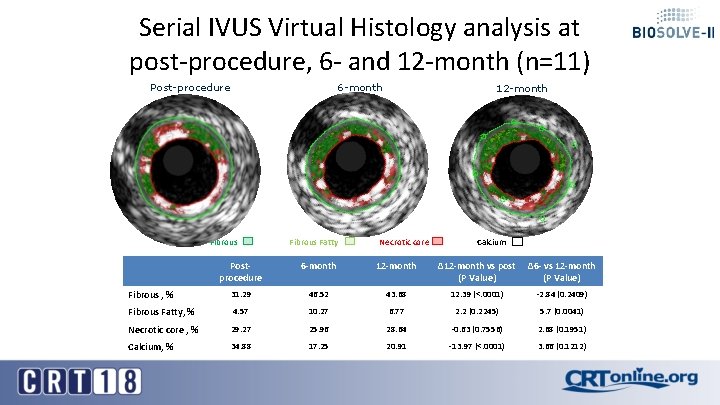
Serial IVUS Virtual Histology analysis at post-procedure, 6 - and 12 -month (n=11) 6 -month Post-procedure Fibrous Fatty 12 -month Necrotic core Calcium Postprocedure 6 -month 12 -month ∆12 -month vs post ∆6 - vs 12 -month (P Value) Fibrous , % 31. 29 46. 52 43. 68 12. 39 (<. 0001) -2. 84 (0. 2409) Fibrous Fatty, % 4. 57 10. 27 6. 77 2. 2 (0. 2245) 5. 7 (0. 0041) Necrotic core , % 29. 27 25. 96 28. 64 -0. 63 (0. 7556) 2. 68 (0. 1951) Calcium, % 34. 88 17. 25 20. 91 -13. 97 (<. 0001) 3. 66 (0. 1212)
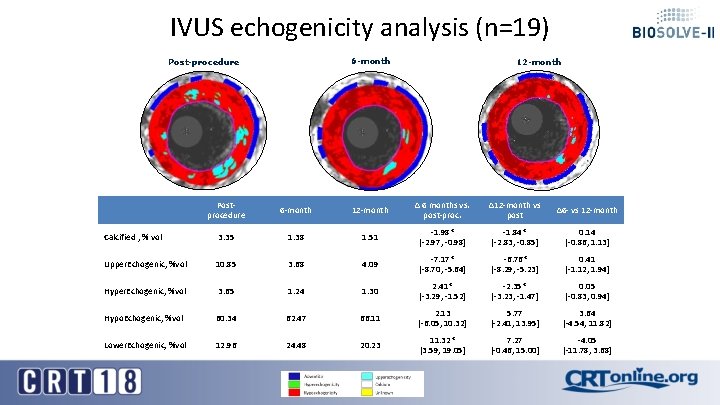
IVUS echogenicity analysis (n=19) 6 -month Post-procedure 12 -month Postprocedure 6 -month 12 -month Δ 6 months vs. post-proc. ∆12 -month vs post ∆6 - vs 12 -month Calcified , % vol 3. 35 1. 38 1. 51 -1. 98* [-2. 97, -0. 98] -1. 84* [-2. 83, -0. 85] 0. 14 [-0. 86, 1. 13] Upper. Echogenic, %vol 10. 85 3. 68 4. 09 -7. 17* [-8. 70, -5. 64] -6. 76* [-8. 29, -5. 23] 0. 41 [-1. 12, 1. 94] Hyper. Echogenic, %vol 3. 65 1. 24 1. 30 2. 41* [-3. 29, -1. 52] -2. 35* [-3. 23, -1. 47] 0. 05 [-0. 83, 0. 94] Hypo. Echogenic, %vol 60. 34 62. 47 66. 11 2. 13 [-6. 05, 10. 32] 5. 77 [-2. 41, 13. 95] 3. 64 [-4. 54, 11. 82] Lower. Echogenic, %vol 12. 96 24. 48 20. 23 11. 32* [3. 59, 19. 05] 7. 27 [-0. 46, 15. 00] -4. 05 [-11. 78, 3. 68]
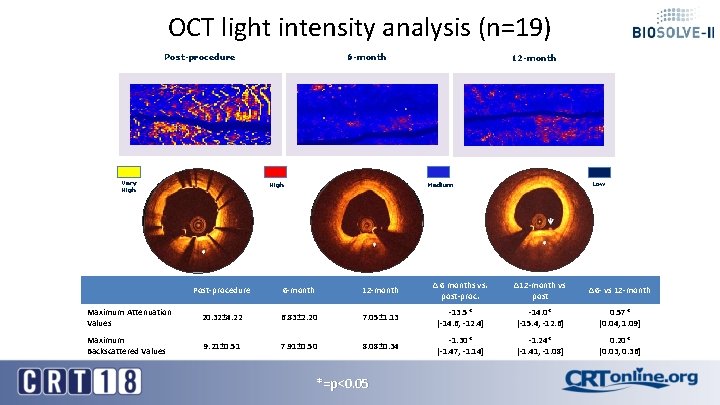
OCT light intensity analysis (n=19) Post-procedure Very High 6 -month High 12 -month Low Medium Post-procedure 6 -month 12 -month Δ 6 months vs. post-proc. ∆12 -month vs post ∆6 - vs 12 -month Maximum Attenuation Values 20. 32± 4. 22 6. 83± 2. 20 7. 05± 1. 13 -13. 5* [-14. 6, -12. 4] -14. 0* [-15. 4, -12. 6] 0. 57* [0. 04, 1. 09] Maximum Backscattered Values 9. 21± 0. 51 7. 91± 0. 50 8. 08± 0. 34 -1. 30* [-1. 47, -1. 14] -1. 24* [-1. 41, -1. 08] 0. 20* [0. 03, 0. 36] *=p<0. 05
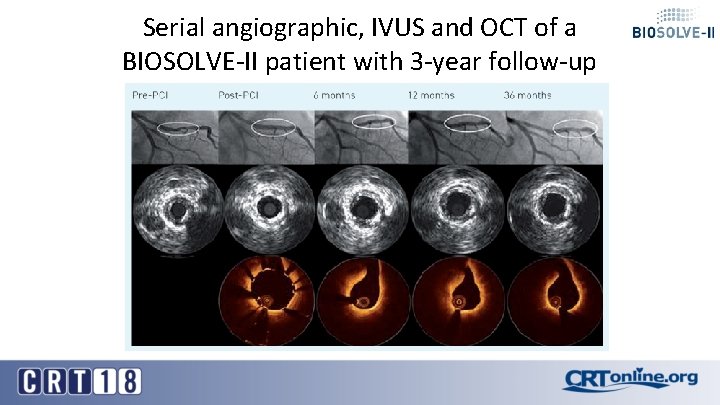
Serial angiographic, IVUS and OCT of a BIOSOLVE-II patient with 3 -year follow-up
Conclusion § TLF (5. 9%) and TLR (3. 3%) rates in BIOSOLVE-II remain low and comparable to other absorbable scaffolds and permanent drug-eluting stents out to 24 -month when the DREAMS-2 G is already fully absorbed § TLF in BIOSOLVE-III confirm the low TLF and TLR rates of the BIOSOLVE-II trial despite more complex lesions being treated in BIOSOLVE-III § There was no definite or probable scaffold thrombosis up to 24 -month FUP with DAPT termination at 12 -month latest § Uncaging of the artery in 80% of cases was already present at 6 months and confirmed after 1 year documented by vasomotion testing § OCT analysis of a patient from BIOSOLVE-II with 3 year follow-up shows no discernable struts any longer and the creation of a «golden tube» already at 12 months with subsequent luminal enlargement.
- Slides: 21