Update on Approved TKIs Jorge Cortes MD Chief




- Slides: 26

Update on Approved TKIs Jorge Cortes, MD Chief, CML and AML Sections Department of Leukemia MD Anderson Cancer Center Houston, Texas

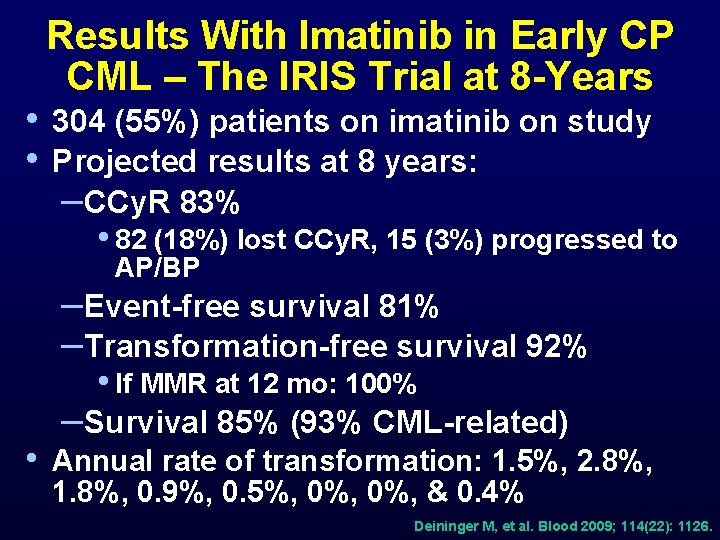
• • Results With Imatinib in Early CP CML – The IRIS Trial at 8 -Years 304 (55%) patients on imatinib on study Projected results at 8 years: –CCy. R 83% • 82 (18%) lost CCy. R, 15 (3%) progressed to AP/BP –Event-free survival 81% –Transformation-free survival 92% • If MMR at 12 mo: 100% • –Survival 85% (93% CML-related) Annual rate of transformation: 1. 5%, 2. 8%, 1. 8%, 0. 9%, 0. 5%, 0%, & 0. 4% Deininger M, et al. Blood 2009; 114(22): 1126.

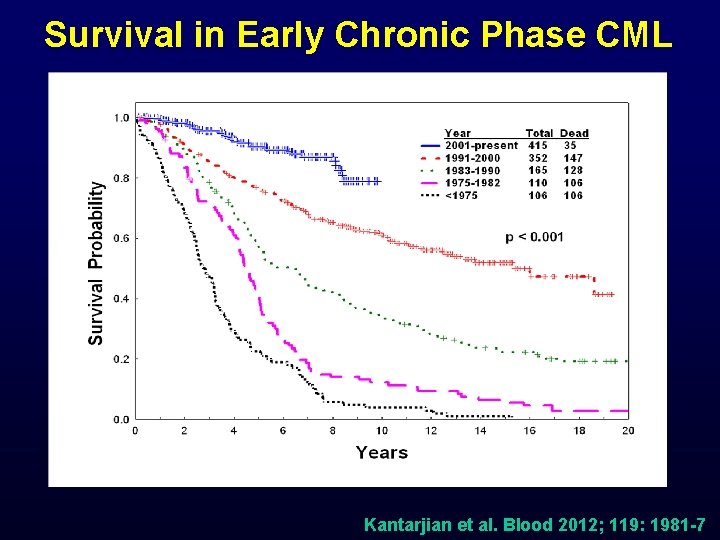
Survival in Early Chronic Phase CML Kantarjian et al. Blood 2012; 119: 1981 -7

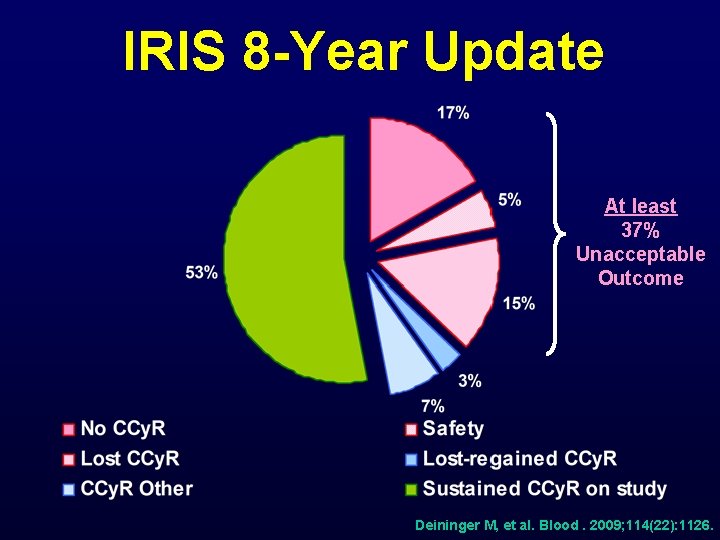
IRIS 8 -Year Update At least 37% Unacceptable Outcome Deininger M, et al. Blood. 2009; 114(22): 1126.

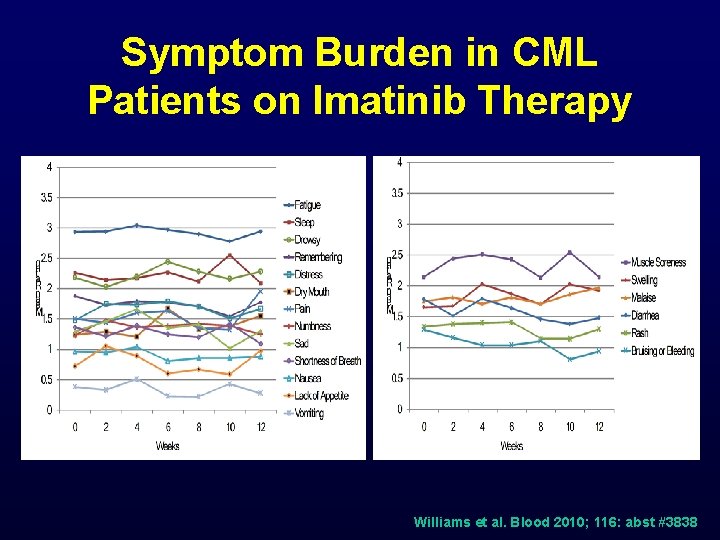
Symptom Burden in CML Patients on Imatinib Therapy Williams et al. Blood 2010; 116: abst #3838

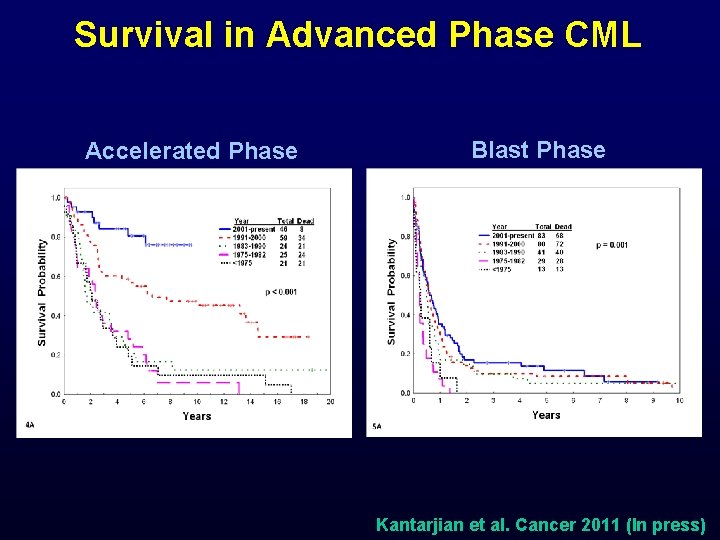
Survival in Advanced Phase CML Accelerated Phase Blast Phase Kantarjian et al. Cancer 2011 (In press)

Improving Frontline Therapy in CML • • Standard-dose imatinib High-dose imatinib Imatinib-based combinations Second generation TKI ─Dasatinib ─Nilotinib ─Bosutinib

• • • Imatinib + IFN as Frontline Therapy for CML IV 1 – 1016 pts randomized to IM 400 mg, IM 800 mg or IM 400 mg + IFNα (median dose 1. 7 MU/d) – No improvement in response rate of 5 -yr PFS with IFN SPIRIT 2 – 636 pts randomized to IM 400 mg, IM 600 mg, IM + ara-C, and IM 400 mg + Peg-IFNα-2 a – Higher rate of CCR, MMR and CMR at 24 mo – No difference in 4 -yr EFS MDACC 3 – 91 pts randomized to IM 800 mg ± Peg-IFN-2 b – No difference in response, EFS, PFS 1 Hehlmann R, et al. JCO 2011 [Epub ahead of print] F, et al. NEJM 2010; 363: 2511 -21 3 Cortes J, et al. Cancer. 2011; 117: 572 -80 2 Guilhot

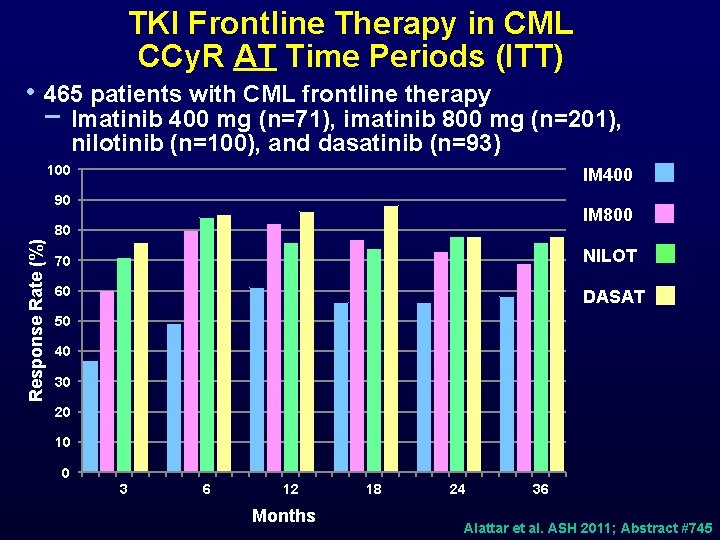
TKI Frontline Therapy in CML CCy. R AT Time Periods (ITT) • 465 patients with CML frontline therapy − Imatinib 400 mg (n=71), imatinib 800 mg (n=201), nilotinib (n=100), and dasatinib (n=93) 100 IM 400 90 IM 800 Response Rate (%) 80 70 NILOT 60 DASAT 50 40 30 20 10 0 3 6 12 Months 18 24 36 Alattar et al. ASH 2011; Abstract #745

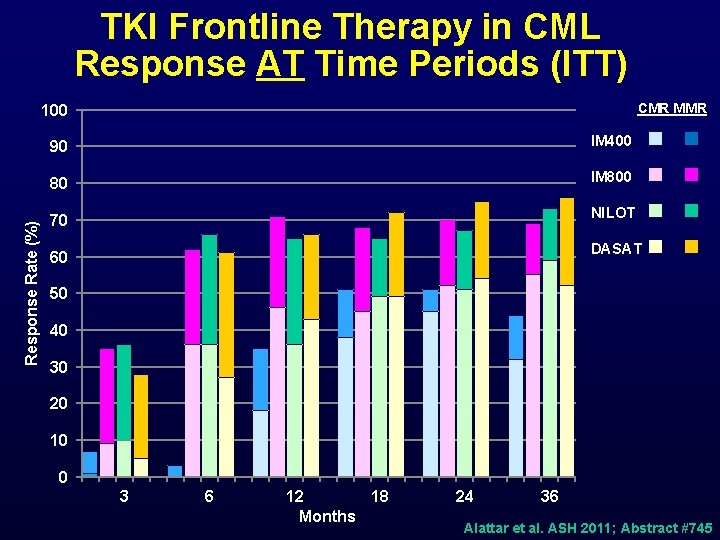
TKI Frontline Therapy in CML Response AT Time Periods (ITT) CMR MMR Response Rate (%) 100 90 IM 400 80 IM 800 70 NILOT DASAT 60 50 40 30 20 10 0 3 6 12 18 Months 24 36 Alattar et al. ASH 2011; Abstract #745

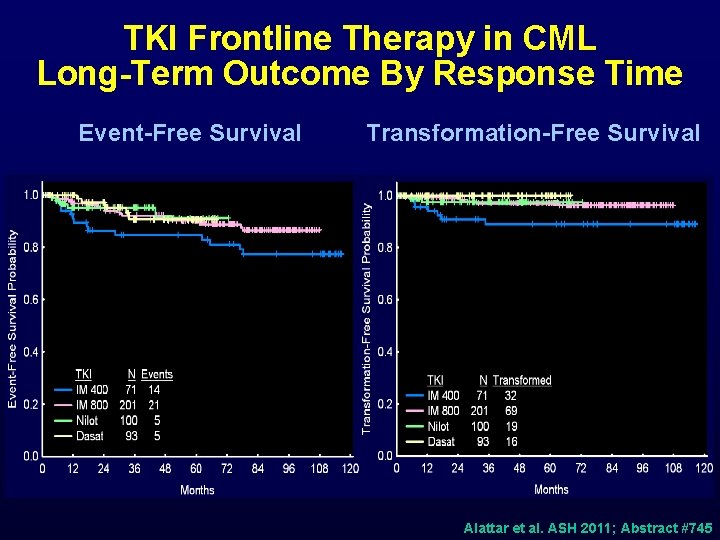
TKI Frontline Therapy in CML Long-Term Outcome By Response Time Event-Free Survival Transformation-Free Survival p<0. 001 Alattar et al. ASH 2011; Abstract #745

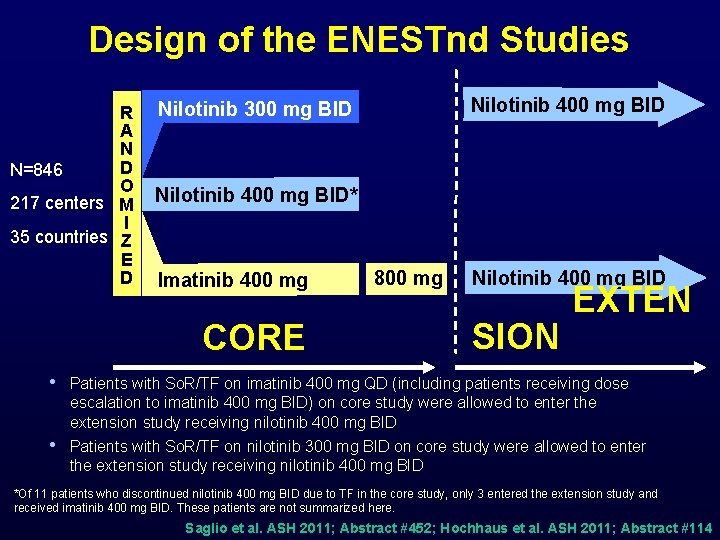
Design of the ENESTnd Studies R A N D N=846 O 217 centers M I 35 countries Z E D Nilotinib 400 mg BID Nilotinib 300 mg BID Nilotinib 400 mg BID* Imatinib 400 mg CORE 800 mg Nilotinib 400 mg BID SION EXTEN • Patients with So. R/TF on imatinib 400 mg QD (including patients receiving dose escalation to imatinib 400 mg BID) on core study were allowed to enter the extension study receiving nilotinib 400 mg BID • Patients with So. R/TF on nilotinib 300 mg BID on core study were allowed to enter the extension study receiving nilotinib 400 mg BID *Of 11 patients who discontinued nilotinib 400 mg BID due to TF in the core study, only 3 entered the extension study and received imatinib 400 mg BID. These patients are not summarized here. Saglio et al. ASH 2011; Abstract #452; Hochhaus et al. ASH 2011; Abstract #114

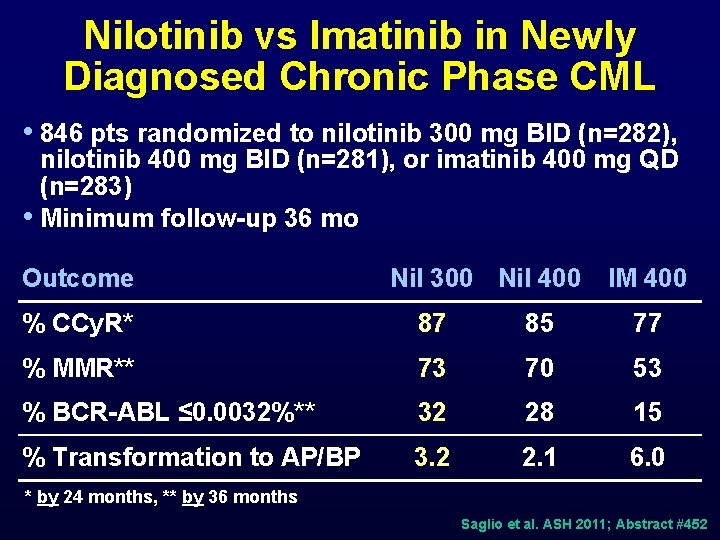
Nilotinib vs Imatinib in Newly Diagnosed Chronic Phase CML • 846 pts randomized to nilotinib 300 mg BID (n=282), nilotinib 400 mg BID (n=281), or imatinib 400 mg QD (n=283) • Minimum follow-up 36 mo Outcome Nil 300 Nil 400 IM 400 % CCy. R* 87 85 77 % MMR** 73 70 53 % BCR-ABL ≤ 0. 0032%** 32 28 15 % Transformation to AP/BP 3. 2 2. 1 6. 0 * by 24 months, ** by 36 months Saglio et al. ASH 2011; Abstract #452

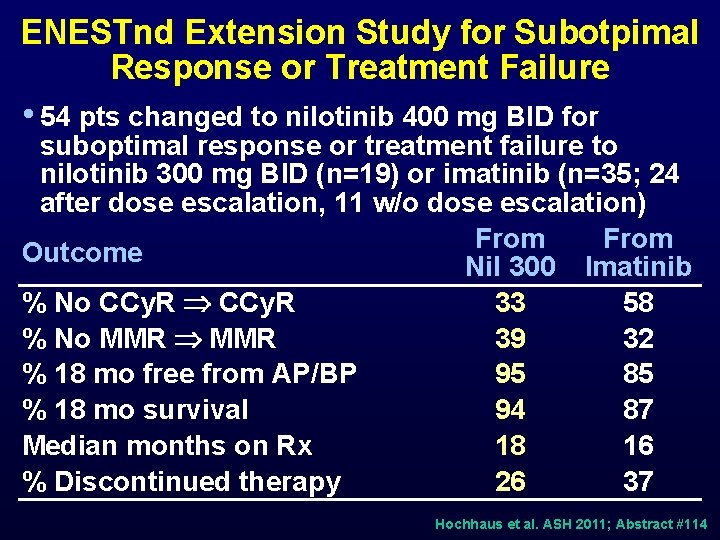
ENESTnd Extension Study for Subotpimal Response or Treatment Failure • 54 pts changed to nilotinib 400 mg BID for suboptimal response or treatment failure to nilotinib 300 mg BID (n=19) or imatinib (n=35; 24 after dose escalation, 11 w/o dose escalation) From Outcome Nil 300 Imatinib % No CCy. R 33 58 % No MMR 39 32 % 18 mo free from AP/BP 95 85 % 18 mo survival 94 87 Median months on Rx 18 16 % Discontinued therapy 26 37 Hochhaus et al. ASH 2011; Abstract #114

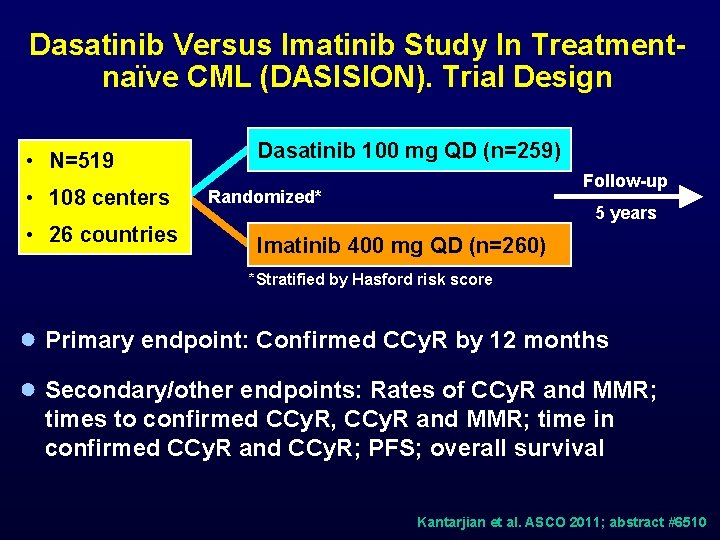
Dasatinib Versus Imatinib Study In Treatmentnaïve CML (DASISION). Trial Design • N=519 • 108 centers • 26 countries Dasatinib 100 mg QD (n=259) Follow-up Randomized* 5 years Imatinib 400 mg QD (n=260) *Stratified by Hasford risk score ● Primary endpoint: Confirmed CCy. R by 12 months ● Secondary/other endpoints: Rates of CCy. R and MMR; times to confirmed CCy. R, CCy. R and MMR; time in confirmed CCy. R and CCy. R; PFS; overall survival Kantarjian et al. ASCO 2011; abstract #6510

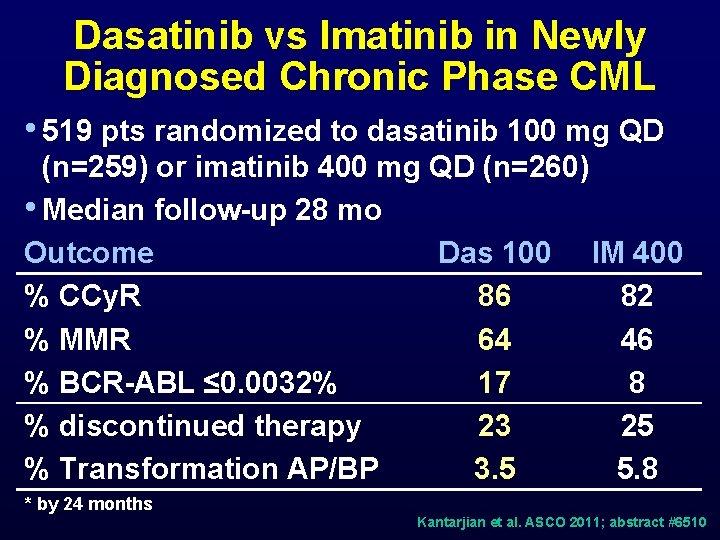
Dasatinib vs Imatinib in Newly Diagnosed Chronic Phase CML • 519 pts randomized to dasatinib 100 mg QD (n=259) or imatinib 400 mg QD (n=260) • Median follow-up 28 mo Outcome Das 100 IM 400 % CCy. R 86 82 % MMR 64 46 % BCR-ABL ≤ 0. 0032% 17 8 % discontinued therapy 23 25 % Transformation AP/BP 3. 5 5. 8 * by 24 months Kantarjian et al. ASCO 2011; abstract #6510

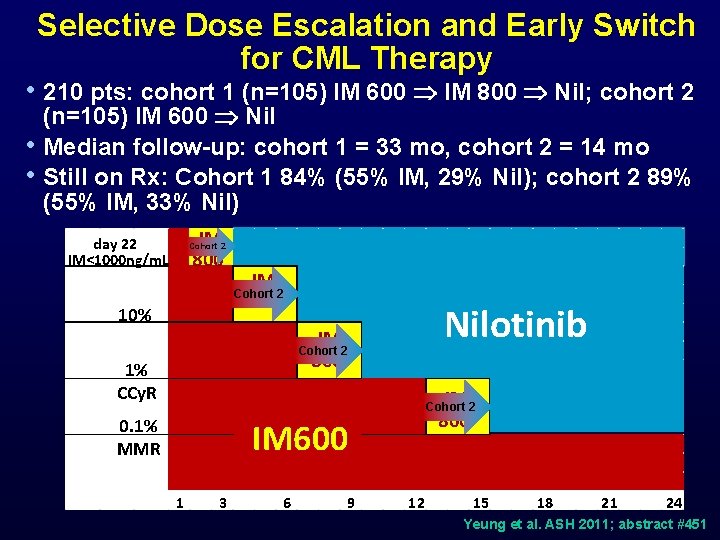
Selective Dose Escalation and Early Switch for CML Therapy • 210 pts: cohort 1 (n=105) IM 600 IM 800 Nil; cohort 2 • • (n=105) IM 600 Nil Median follow-up: cohort 1 = 33 mo, cohort 2 = 14 mo Still on Rx: Cohort 1 84% (55% IM, 29% Nil); cohort 2 89% (55% IM, 33% Nil) IM 800 day 22 IM<1000 ng/m. L Cohort 2 IM 800 Cohort 2 10% Nilotinib IM Cohort 2 800 1% CCy. R IM 800 Cohort 2 0. 1% MMR IM 600 1 3 6 9 12 15 18 21 24 Yeung et al. ASH 2011; abstract #451

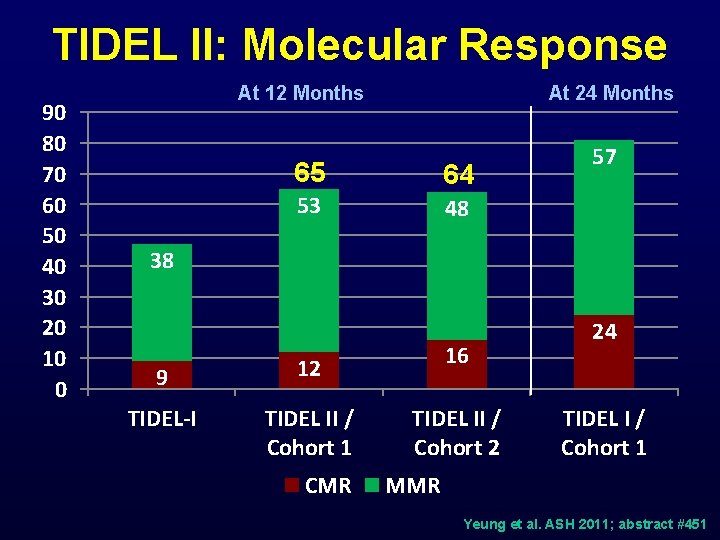
TIDEL II: Molecular Response 90 80 70 60 50 40 30 20 10 0 At 12 Months At 24 Months 65 64 53 48 57 38 9 12 TIDEL-I TIDEL II / Cohort 1 CMR 16 TIDEL II / Cohort 2 24 TIDEL I / Cohort 1 MMR Yeung et al. ASH 2011; abstract #451

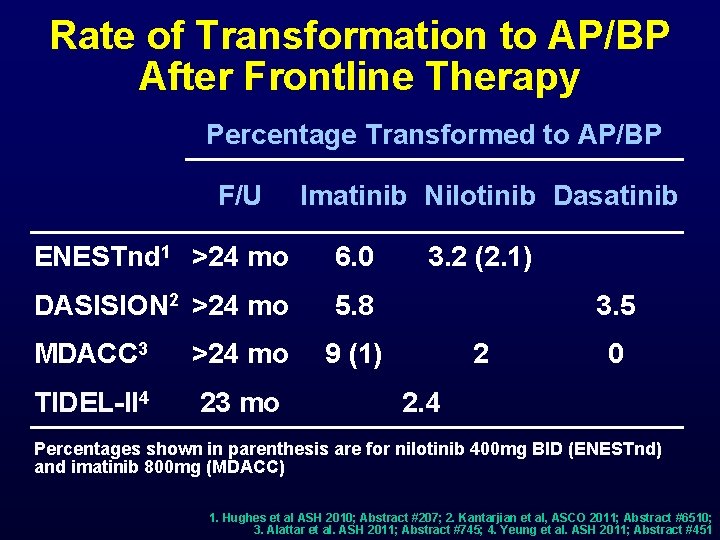
Rate of Transformation to AP/BP After Frontline Therapy Percentage Transformed to AP/BP F/U Imatinib Nilotinib Dasatinib ENESTnd 1 >24 mo 6. 0 DASISION 2 >24 mo 5. 8 MDACC 3 >24 mo TIDEL-II 4 23 mo 3. 2 (2. 1) 3. 5 9 (1) 2 0 2. 4 Percentages shown in parenthesis are for nilotinib 400 mg BID (ENESTnd) and imatinib 800 mg (MDACC) 1. Hughes et al ASH 2010; Abstract #207; 2. Kantarjian et al, ASCO 2011; Abstract #6510; 3. Alattar et al. ASH 2011; Abstract #745; 4. Yeung et al. ASH 2011; Abstract #451

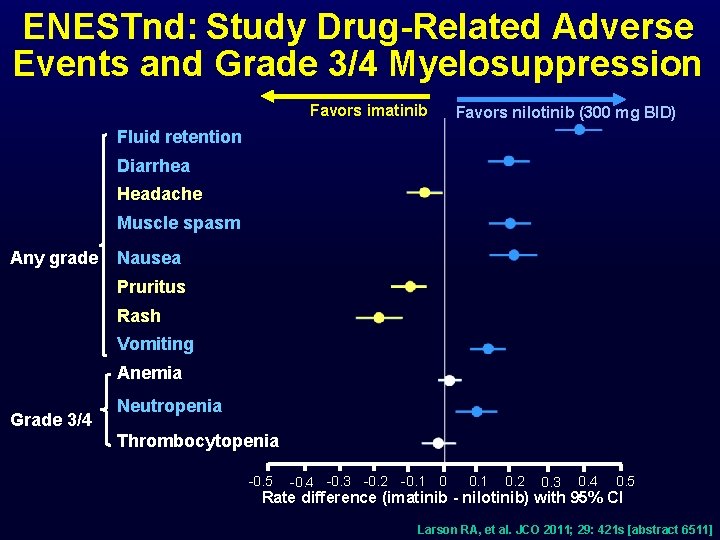
ENESTnd: Study Drug-Related Adverse Events and Grade 3/4 Myelosuppression Favors imatinib Favors nilotinib (300 mg BID) Fluid retention Diarrhea Headache Muscle spasm Any grade Nausea Pruritus Rash Vomiting Anemia Grade 3/4 Neutropenia Thrombocytopenia -0. 5 -0. 4 -0. 3 -0. 2 -0. 1 0. 2 0. 3 0. 4 0. 5 Rate difference (imatinib - nilotinib) with 95% CI Larson RA, et al. JCO 2011; 29: 421 s [abstract 6511]

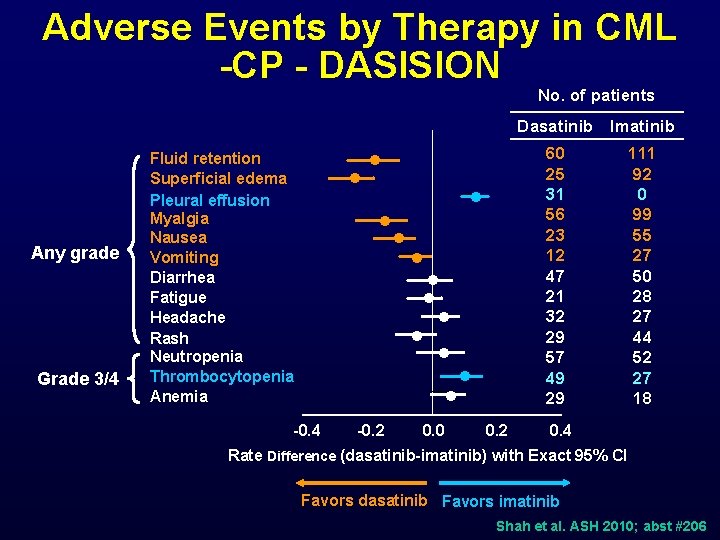
Adverse Events by Therapy in CML -CP - DASISION No. of patients Dasatinib Imatinib Any grade Grade 3/4 Fluid retention Superficial edema Pleural effusion Myalgia Nausea Vomiting Diarrhea Fatigue Headache Rash Neutropenia Thrombocytopenia Anemia l 60 25 31 56 23 12 47 21 32 29 57 49 29 l l l -0. 4 -0. 2 0. 0 0. 2 111 92 0 99 55 27 50 28 27 44 52 27 18 0. 4 Rate Difference (dasatinib-imatinib) with Exact 95% CI Favors dasatinib Favors imatinib Shah et al. ASH 2010; abst #206

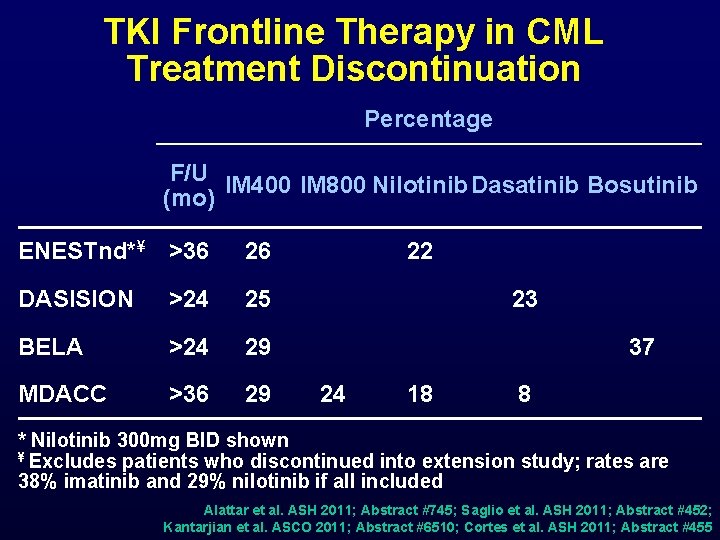
TKI Frontline Therapy in CML Treatment Discontinuation Percentage F/U IM 400 IM 800 Nilotinib Dasatinib Bosutinib (mo) ENESTnd*¥ >36 26 DASISION >24 25 BELA >24 29 MDACC >36 29 22 23 37 24 18 8 * Nilotinib 300 mg BID shown ¥ Excludes patients who discontinued into extension study; rates are 38% imatinib and 29% nilotinib if all included Alattar et al. ASH 2011; Abstract #745; Saglio et al. ASH 2011; Abstract #452; Kantarjian et al. ASCO 2011; Abstract #6510; Cortes et al. ASH 2011; Abstract #455

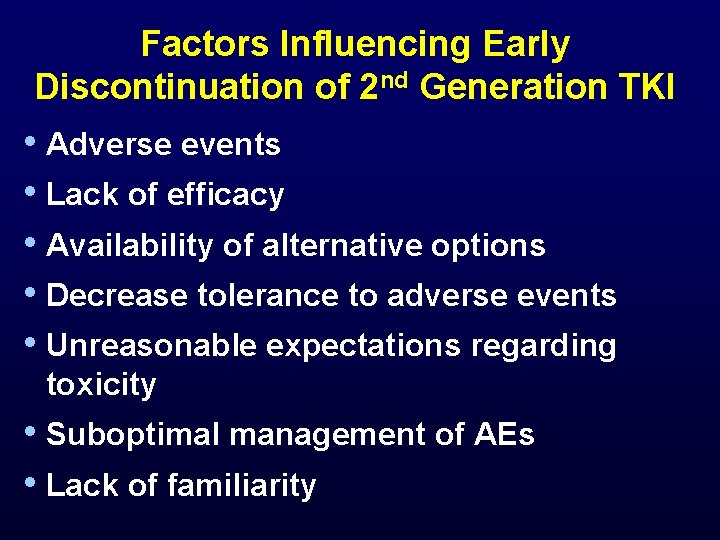
Factors Influencing Early Discontinuation of 2 nd Generation TKI • Adverse events • Lack of efficacy • Availability of alternative options • Decrease tolerance to adverse events • Unreasonable expectations regarding toxicity • Suboptimal management of AEs • Lack of familiarity

Take Home Message – CML 2012 ¼ • Frontline therapy: new standard • Imatinib is good • 2 nd generation TKI (dasatinib, nilotinib, bosutinib? ) probably better • Earlier responses • Possible improvement EFS, TFS • Best drug vs best strategy? • Sequential therapy? • Adequate management is vital

Now in its third year the i. CMLf has already achieved a great deal: • Over 40 practicing hematologists (preceptors) have attended educational programs at internationally renowned CML centres of excellence • Through the i. CMLf Virtual Education Program the i. CMLf has provided in excess of 5500 people access to up to date online CML education sessions from leading experts

Questions? jcortes@mdanderson. org 713 -794 -5783