Update on Antiretroviral Therapy Current Treatment Guidelines and

















- Slides: 50

Update on Antiretroviral Therapy: Current Treatment Guidelines and What’s on the Horizon Where Do We Come From? What Are We? Where Are We Going? – Paul Gauguin Rajesh T. Gandhi, M. D. Disclosures: grant support from Gilead, Roche, EBSCO Thanks to Jacqueline Chu, M. D. , Joe Eron, M. D. and Neha Patel for assistance with slides

Updated: July 28, 2015 Updated: Sept. 2015 http: //www. aidsinfo. nih. gov/Content. Files/Adultand. Adolescent. GL. pdf

Case • 55 yo M with HIV infection • Past history: gastroesophageal reflux disease (GERD), allergic rhinitis, hypertension, smoking, elevated lipids • Medications: fluticasone, omeprazole • CD 4 cell count 550. HIV RNA 125, 000 • He asks 3 questions: – “When should I be treated for HIV? ” – “What should l be treated with? ” – “If I take medicines, will I be cured? ”

When to Start

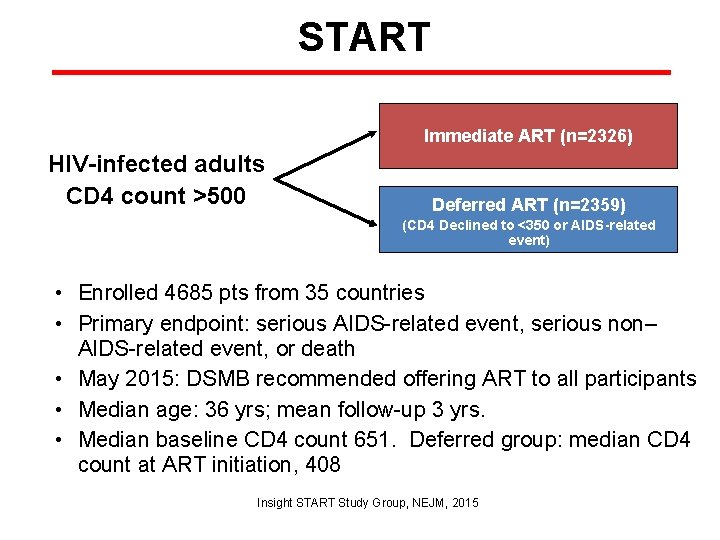
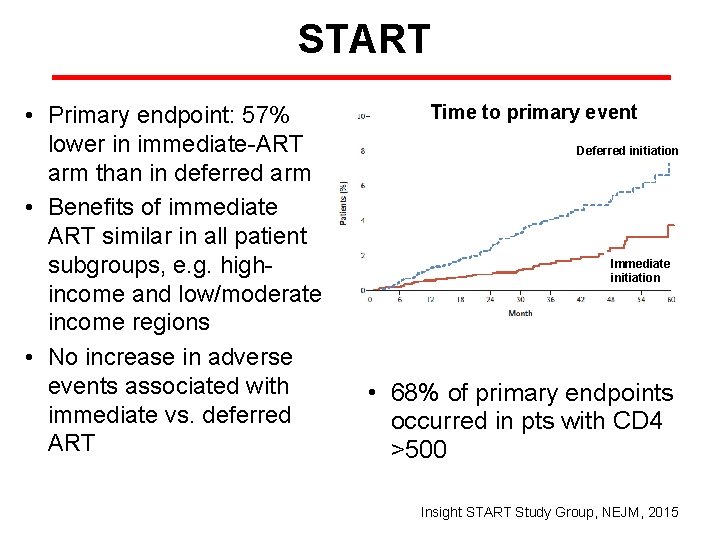
START Immediate ART (n=2326) HIV-infected adults CD 4 count >500 Deferred ART (n=2359) (CD 4 Declined to <350 or AIDS-related event) • Enrolled 4685 pts from 35 countries • Primary endpoint: serious AIDS-related event, serious non– AIDS-related event, or death • May 2015: DSMB recommended offering ART to all participants • Median age: 36 yrs; mean follow-up 3 yrs. • Median baseline CD 4 count 651. Deferred group: median CD 4 count at ART initiation, 408 Insight START Study Group, NEJM, 2015

START • Primary endpoint: 57% lower in immediate-ART arm than in deferred arm • Benefits of immediate ART similar in all patient subgroups, e. g. highincome and low/moderate income regions • No increase in adverse events associated with immediate vs. deferred ART Time to primary event Deferred initiation Immediate initiation • 68% of primary endpoints occurred in pts with CD 4 >500 Insight START Study Group, NEJM, 2015

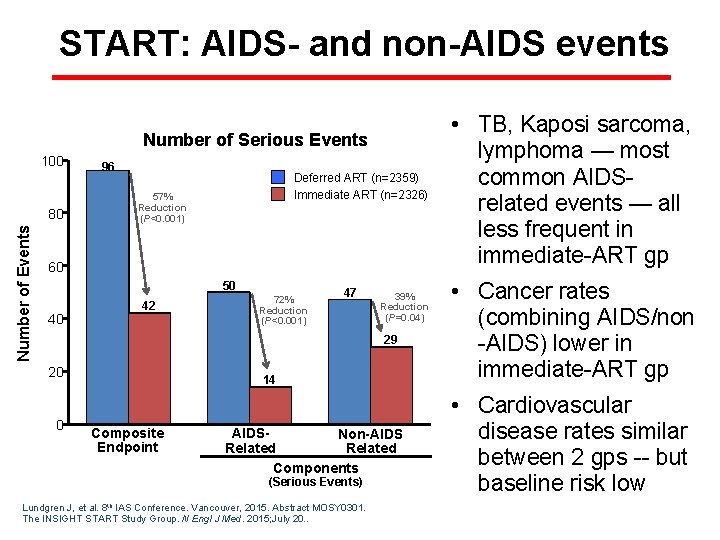
START: AIDS- and non-AIDS events Number of Serious Events 100 Number of Events 80 96 Deferred ART (n=2359) Immediate ART (n=2326) 57% Reduction (P<0. 001) 60 50 40 42 72% Reduction (P<0. 001) 47 39% Reduction (P=0. 04) 29 20 0 14 Composite Endpoint AIDSNon-AIDS Related Components (Serious Events) Lundgren J, et al. 8 th IAS Conference. Vancouver, 2015. Abstract MOSY 0301. The INSIGHT START Study Group. N Engl J Med. 2015; July 20. . • TB, Kaposi sarcoma, lymphoma — most common AIDSrelated events — all less frequent in immediate-ART gp • Cancer rates (combining AIDS/non -AIDS) lower in immediate-ART gp • Cardiovascular disease rates similar between 2 gps -- but baseline risk low

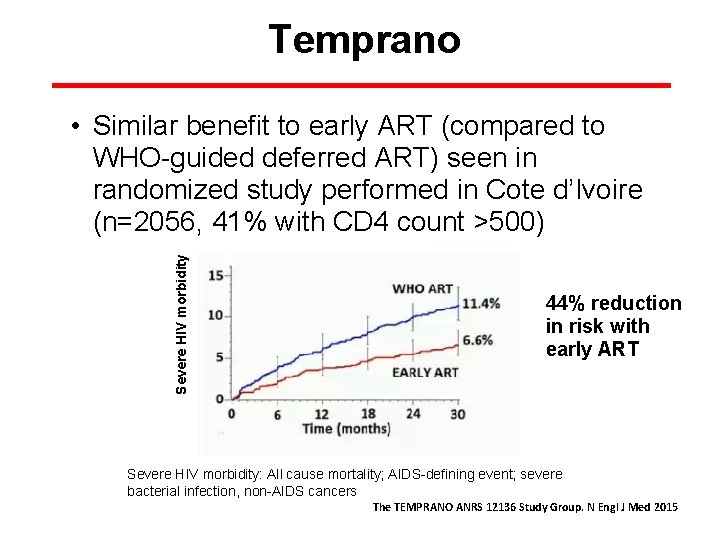
Temprano Severe HIV morbidity • Similar benefit to early ART (compared to WHO-guided deferred ART) seen in randomized study performed in Cote d’Ivoire (n=2056, 41% with CD 4 count >500) 44% reduction in risk with early ART Severe HIV morbidity: All cause mortality; AIDS-defining event; severe bacterial infection, non-AIDS cancers The TEMPRANO ANRS 12136 Study Group. N Engl J Med 2015

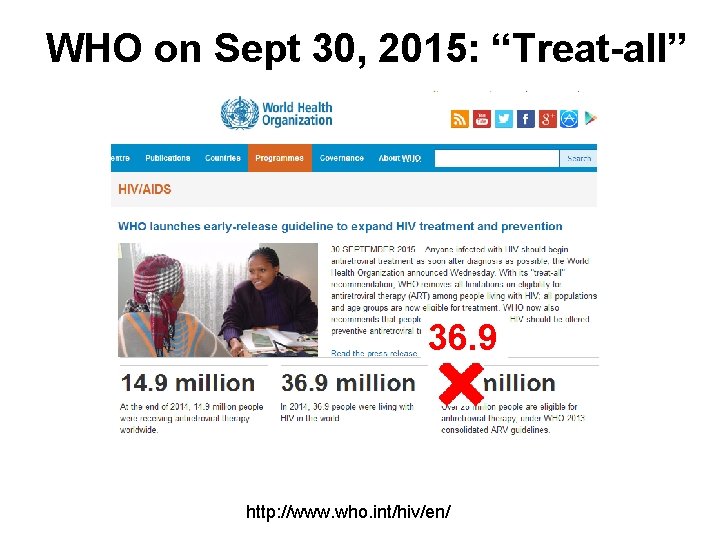
WHO on Sept 30, 2015: “Treat-all” 36. 9 http: //www. who. int/hiv/en/

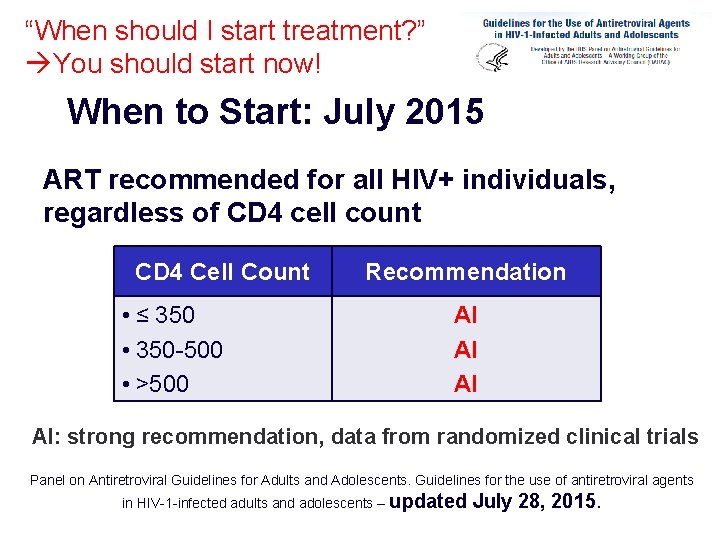
“When should I start treatment? ” You should start now! When to Start: July 2015 ART recommended for all HIV+ individuals, regardless of CD 4 cell count CD 4 Cell Count • ≤ 350 • 350 -500 • >500 Recommendation AI AI: strong recommendation, data from randomized clinical trials Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents – updated US DHHS Guidelines, January 2011. July 28, 2015.

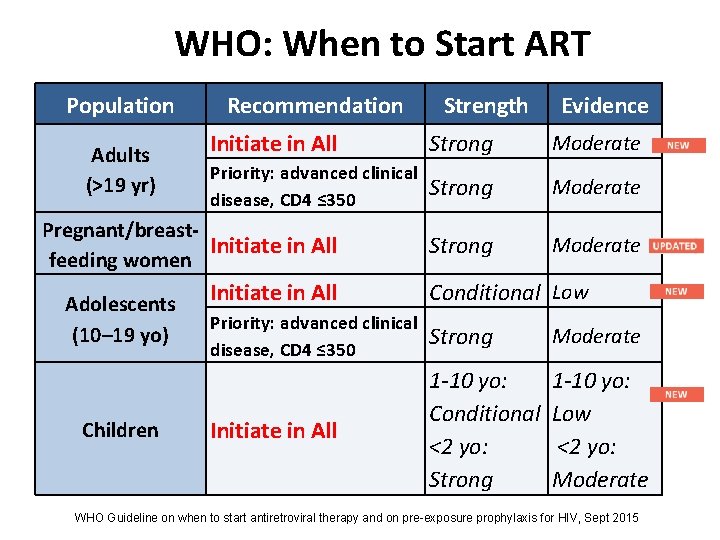
WHO: When to Start ART Population Adults (>19 yr) Recommendation Children Evidence Initiate in All Strong Moderate Priority: advanced clinical disease, CD 4 ≤ 350 Strong Moderate Pregnant/breast. Initiate in All feeding women Adolescents (10– 19 yo) Strength Initiate in All Conditional Low Priority: advanced clinical disease, CD 4 ≤ 350 Strong Moderate Initiate in All 1 -10 yo: Conditional <2 yo: Strong 1 -10 yo: Low <2 yo: Moderate WHO Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV, Sept 2015

When to Start ART after Opportunistic Infection (OI) OI When to start Cryptosporidiosis, microsporidiosis, PML As part of initial therapy of the OI PCP, MAC, Toxoplasma, most other OIs Within 2 weeks Tuberculosis If CD 4 count <50: within 2 wk If CD 4 count >50: within 8 -12 wks (TB meningitis: careful monitoring/consultation) Cryptococcal meningitis 4 -5 weeks after initiation of anti-fungal therapy Zolopa A et al, PLo. S One. 2009; Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents, 2013, WHO Consolidated ARV Guidelines, 2013. Zanoni and Gandhi, Inf Dis Clin N Am, 2014

“How should I be treated? ” What to Start http: //www. aidsinfo. nih. gov/Content. Files/Adultand. Adolescent. GL. pdf 13

Case – What to Start? • 55 yo M with HIV • GERD, allergic rhinitis, HTN, smoking, elevated lipids. Medications: fluticasone, omeprazole • CD 4 cell count 550. HIV RNA 125, 000

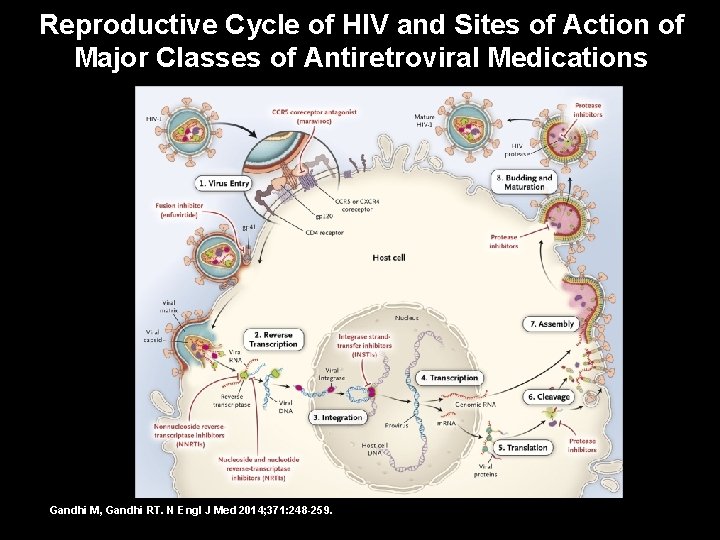
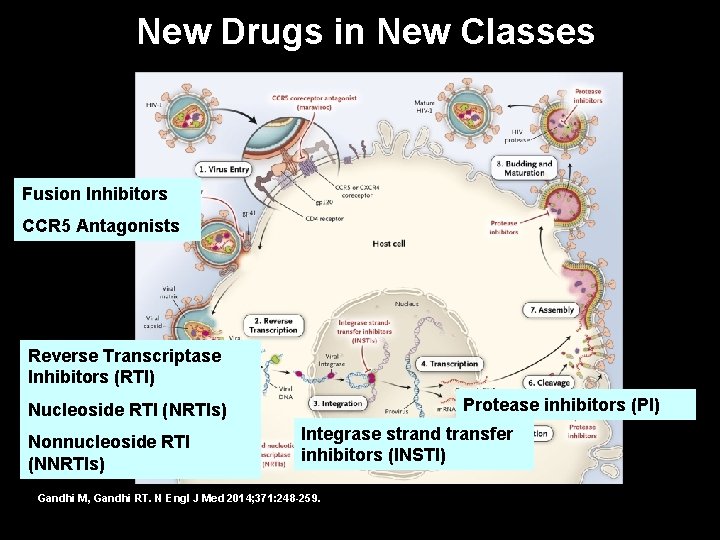
Reproductive Cycle of HIV and Sites of Action of Major Classes of Antiretroviral Medications Gandhi M, Gandhi RT. N Engl J Med 2014; 371: 248 -259.

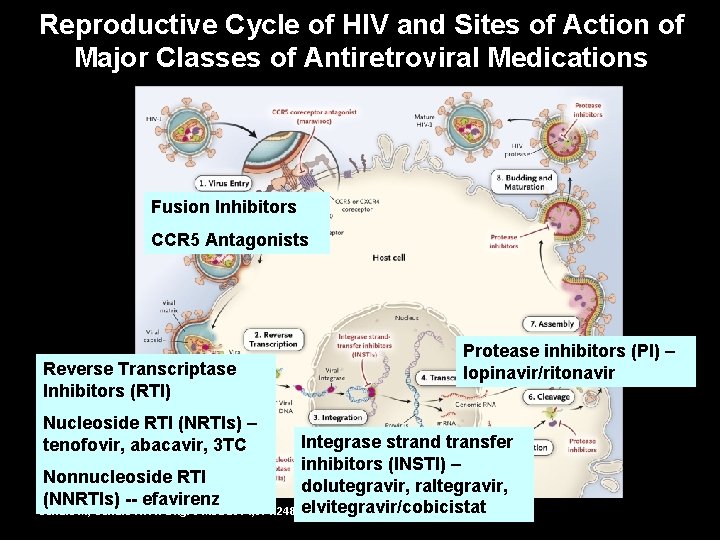
Reproductive Cycle of HIV and Sites of Action of Major Classes of Antiretroviral Medications Fusion Inhibitors CCR 5 Antagonists Reverse Transcriptase Inhibitors (RTI) Nucleoside RTI (NRTIs) – tenofovir, abacavir, 3 TC Protease inhibitors (PI) – lopinavir/ritonavir Integrase strand transfer inhibitors (INSTI) – Nonnucleoside RTI dolutegravir, raltegravir, (NNRTIs) -- efavirenz elvitegravir/cobicistat Gandhi M, Gandhi RT. N Engl J Med 2014; 371: 248 -259.

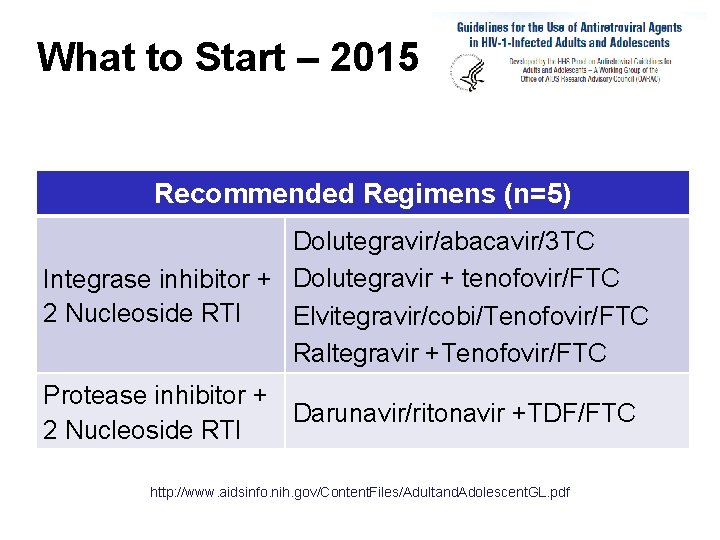
What to Start – 2015 Recommended Regimens (n=5) Dolutegravir/abacavir/3 TC Integrase inhibitor + Dolutegravir + tenofovir/FTC 2 Nucleoside RTI Elvitegravir/cobi/Tenofovir/FTC Raltegravir +Tenofovir/FTC Protease inhibitor + Darunavir/ritonavir +TDF/FTC 2 Nucleoside RTI http: //www. aidsinfo. nih. gov/Content. Files/Adultand. Adolescent. GL. pdf

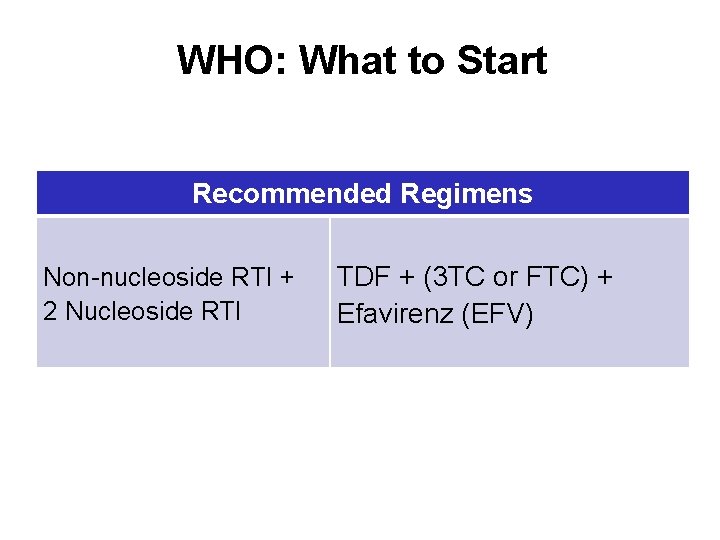
WHO: What to Start Recommended Regimens Non-nucleoside RTI + 2 Nucleoside RTI TDF + (3 TC or FTC) + Efavirenz (EFV)

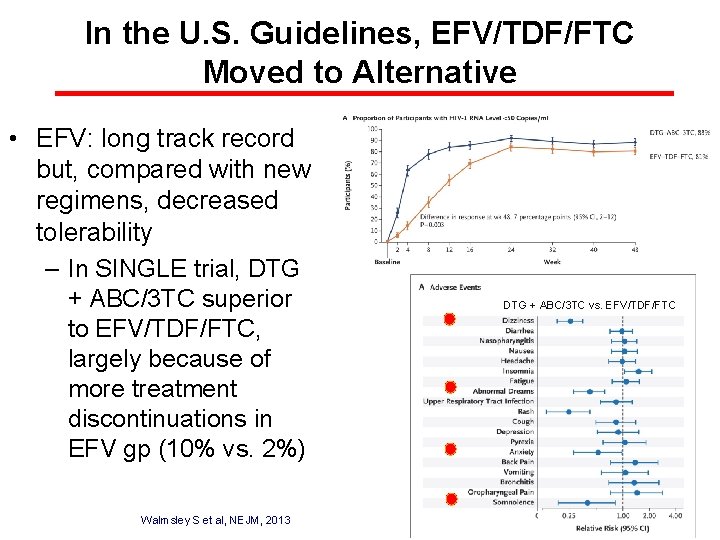
In the U. S. Guidelines, EFV/TDF/FTC Moved to Alternative • EFV: long track record but, compared with new regimens, decreased tolerability – In SINGLE trial, DTG + ABC/3 TC superior to EFV/TDF/FTC, largely because of more treatment discontinuations in EFV gp (10% vs. 2%) Walmsley S et al, NEJM, 2013 DTG + ABC/3 TC vs. EFV/TDF/FTC

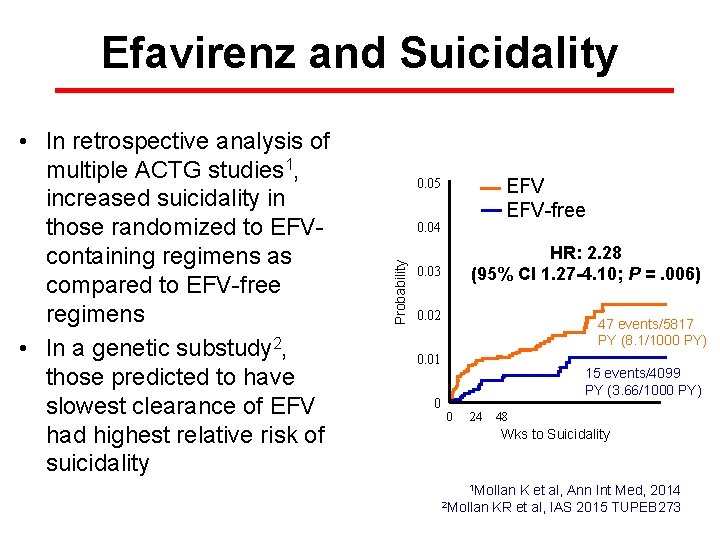
Efavirenz and Suicidality EFV-free 0. 05 0. 04 Probability • In retrospective analysis of multiple ACTG studies 1, increased suicidality in those randomized to EFVcontaining regimens as compared to EFV-free regimens • In a genetic substudy 2, those predicted to have slowest clearance of EFV had highest relative risk of suicidality HR: 2. 28 (95% CI 1. 27 -4. 10; P =. 006) 0. 03 0. 02 47 events/5817 PY (8. 1/1000 PY) 0. 01 0 15 events/4099 PY (3. 66/1000 PY) 0 24 48 72 96 120 144 168 192 Wks to Suicidality 1 Mollan K et al, Ann Int Med, 2014 2 Mollan KR et al, IAS 2015 TUPEB 273

Integrase Inhibitors for 1 st Line HIV Therapy • Elvitegravir/cobi superior to atazanavir/ritonavir (PI) in HIV+ women (WAVES) • Raltegravir superior to atazanavir/ritonavir and darunavir/ritonavir (ACTG A 5257) • Dolutegravir superior to darunavir/ritonavir (FLAMINGO) • Dolutegravir superior to efavirenz (SINGLE) In U. S. guidelines, 4 of 5 recommended options for initial therapy include integrase inhibitor


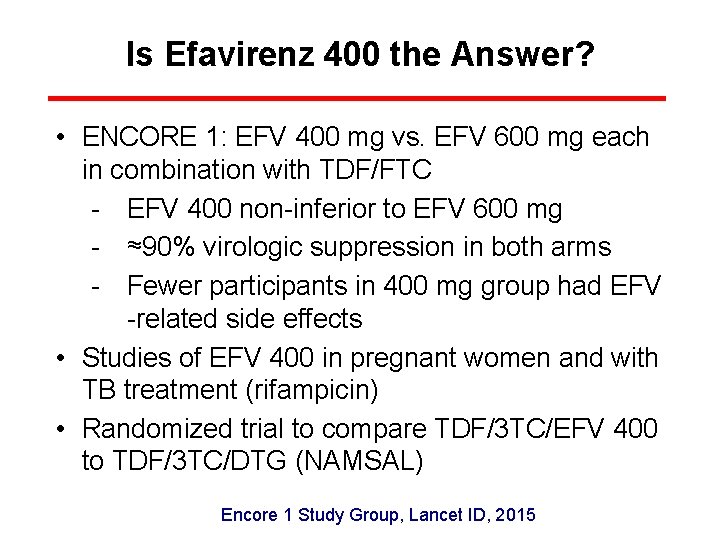
Is Efavirenz 400 the Answer? • ENCORE 1: EFV 400 mg vs. EFV 600 mg each in combination with TDF/FTC - EFV 400 non-inferior to EFV 600 mg - ≈90% virologic suppression in both arms - Fewer participants in 400 mg group had EFV -related side effects • Studies of EFV 400 in pregnant women and with TB treatment (rifampicin) • Randomized trial to compare TDF/3 TC/EFV 400 to TDF/3 TC/DTG (NAMSAL) Encore 1 Study Group, Lancet ID, 2015

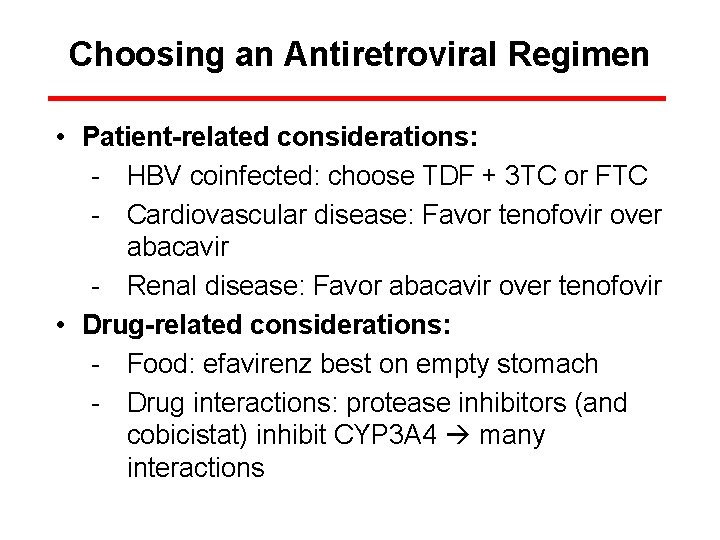
Choosing an Antiretroviral Regimen • Patient-related considerations: - HBV coinfected: choose TDF + 3 TC or FTC - Cardiovascular disease: Favor tenofovir over abacavir - Renal disease: Favor abacavir over tenofovir • Drug-related considerations: - Food: efavirenz best on empty stomach - Drug interactions: protease inhibitors (and cobicistat) inhibit CYP 3 A 4 many interactions

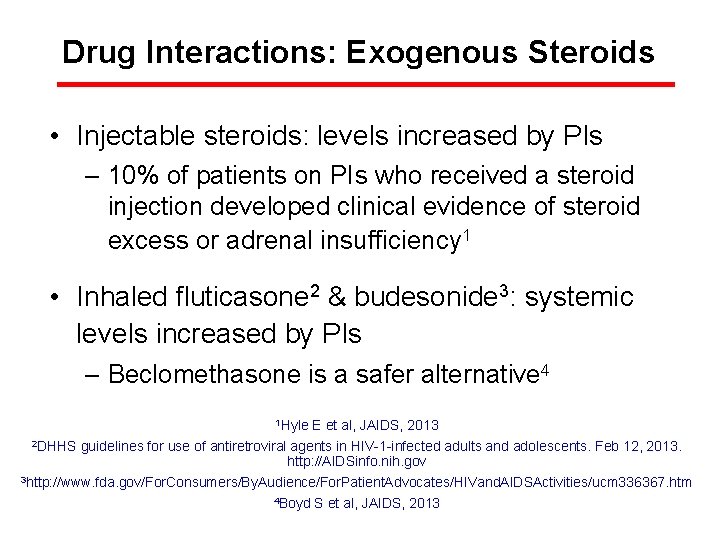
Drug Interactions: Exogenous Steroids • Injectable steroids: levels increased by PIs – 10% of patients on PIs who received a steroid injection developed clinical evidence of steroid excess or adrenal insufficiency 1 • Inhaled fluticasone 2 & budesonide 3: systemic levels increased by PIs – Beclomethasone is a safer alternative 4 1 Hyle E et al, JAIDS, 2013 2 DHHS guidelines for use of antiretroviral agents in HIV-1 -infected adults and adolescents. Feb 12, 2013. http: //AIDSinfo. nih. gov 3 http: //www. fda. gov/For. Consumers/By. Audience/For. Patient. Advocates/HIVand. AIDSActivities/ucm 336367. htm 4 Boyd S et al, JAIDS, 2013

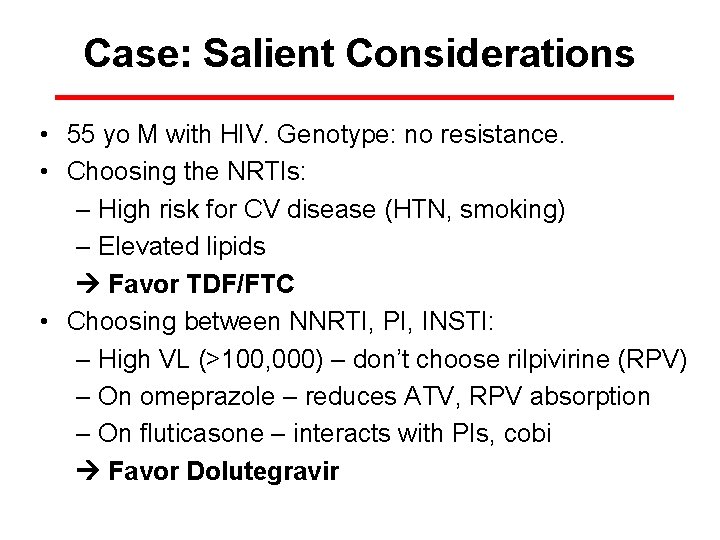
Case: Salient Considerations • 55 yo M with HIV. Genotype: no resistance. • Choosing the NRTIs: – High risk for CV disease (HTN, smoking) – Elevated lipids Favor TDF/FTC • Choosing between NNRTI, PI, INSTI: – High VL (>100, 000) – don’t choose rilpivirine (RPV) – On omeprazole – reduces ATV, RPV absorption – On fluticasone – interacts with PIs, cobi Favor Dolutegravir

ART: On the Horizon

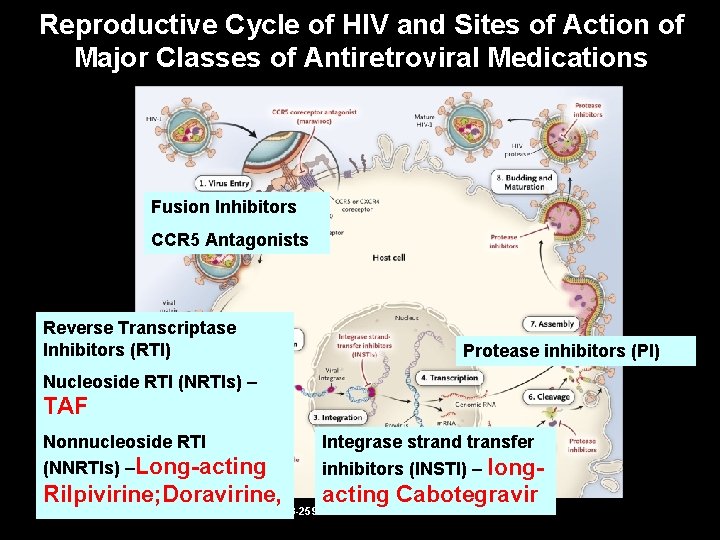
Reproductive Cycle of HIV and Sites of Action of Major Classes of Antiretroviral Medications Fusion Inhibitors CCR 5 Antagonists Reverse Transcriptase Inhibitors (RTI) Protease inhibitors (PI) Nucleoside RTI (NRTIs) – TAF Nonnucleoside RTI (NNRTIs) –Long-acting Integrase strand transfer inhibitors (INSTI) – long- Rilpivirine; Doravirine, acting Cabotegravir Gandhi M, Gandhi RT. N Engl J Med 2014; 371: 248 -259.

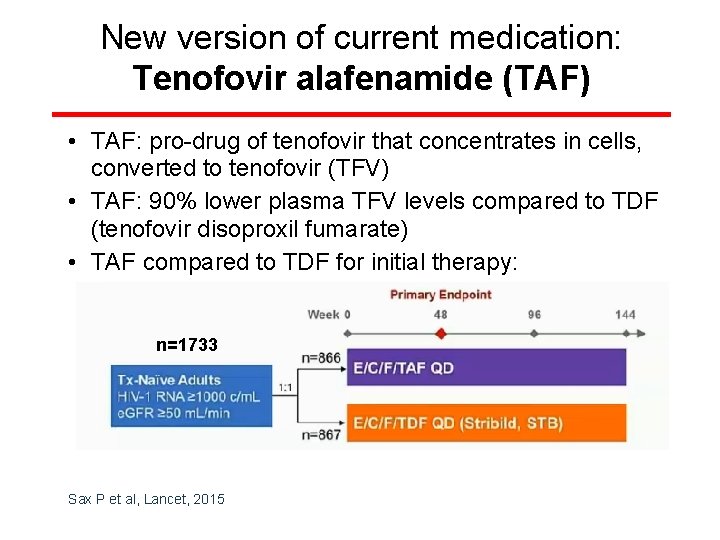
New version of current medication: Tenofovir alafenamide (TAF) • TAF: pro-drug of tenofovir that concentrates in cells, converted to tenofovir (TFV) • TAF: 90% lower plasma TFV levels compared to TDF (tenofovir disoproxil fumarate) • TAF compared to TDF for initial therapy: n=1733 Sax P et al, Lancet, 2015

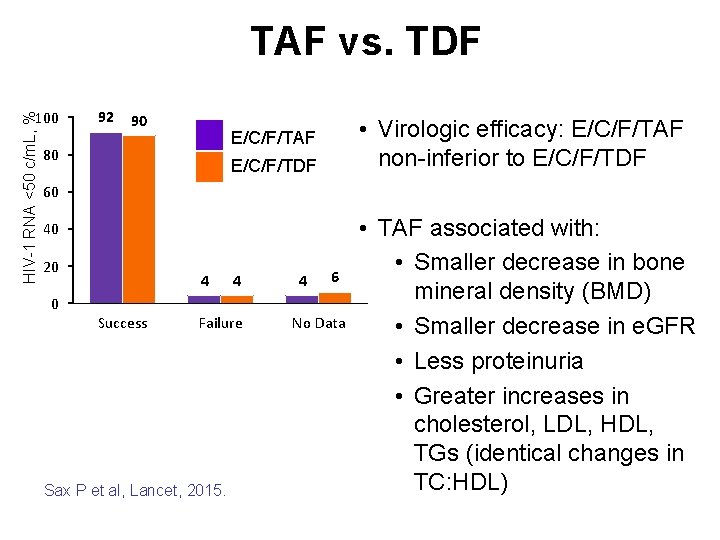
HIV-1 RNA <50 c/m. L, % TAF vs. TDF 100 92 90 • Virologic efficacy: E/C/F/TAF non-inferior to E/C/F/TDF E/C/F/TAF 80 E/C/F/TDF 60 40 20 4 4 4 6 0 Success Failure Sax P et al, Lancet, 2015. No Data • TAF associated with: • Smaller decrease in bone mineral density (BMD) • Smaller decrease in e. GFR • Less proteinuria • Greater increases in cholesterol, LDL, HDL, TGs (identical changes in TC: HDL)

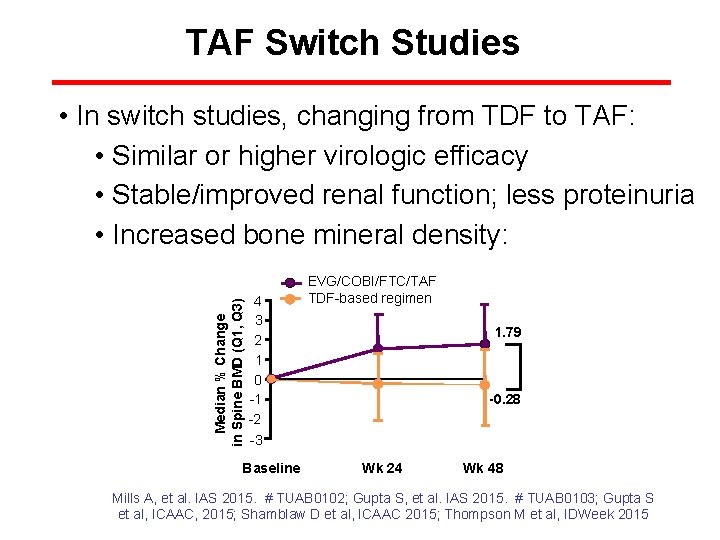
TAF Switch Studies Median % Change in Spine BMD (Q 1, Q 3) • In switch studies, changing from TDF to TAF: • Similar or higher virologic efficacy • Stable/improved renal function; less proteinuria • Increased bone mineral density: 4 3 2 1 0 -1 -2 -3 Baseline EVG/COBI/FTC/TAF TDF-based regimen 1. 79 -0. 28 Wk 24 Wk 48 Mills A, et al. IAS 2015. # TUAB 0102; Gupta S, et al. IAS 2015. # TUAB 0103; Gupta S et al, ICAAC, 2015; Shamblaw D et al, ICAAC 2015; Thompson M et al, IDWeek 2015

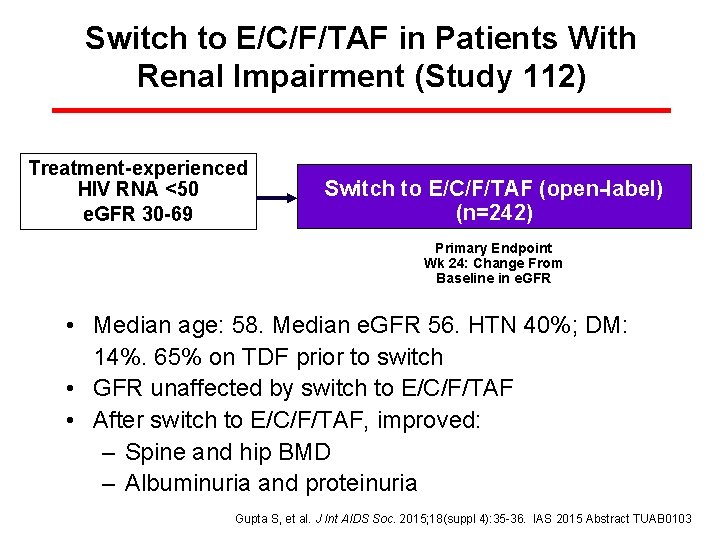
Switch to E/C/F/TAF in Patients With Renal Impairment (Study 112) Treatment-experienced HIV RNA <50 e. GFR 30 -69 Switch to E/C/F/TAF (open-label) (n=242) Primary Endpoint Wk 24: Change From Baseline in e. GFR • Median age: 58. Median e. GFR 56. HTN 40%; DM: 14%. 65% on TDF prior to switch • GFR unaffected by switch to E/C/F/TAF • After switch to E/C/F/TAF, improved: – Spine and hip BMD – Albuminuria and proteinuria Gupta S, et al. J Int AIDS Soc. 2015; 18(suppl 4): 35 -36. IAS 2015 Abstract TUAB 0103

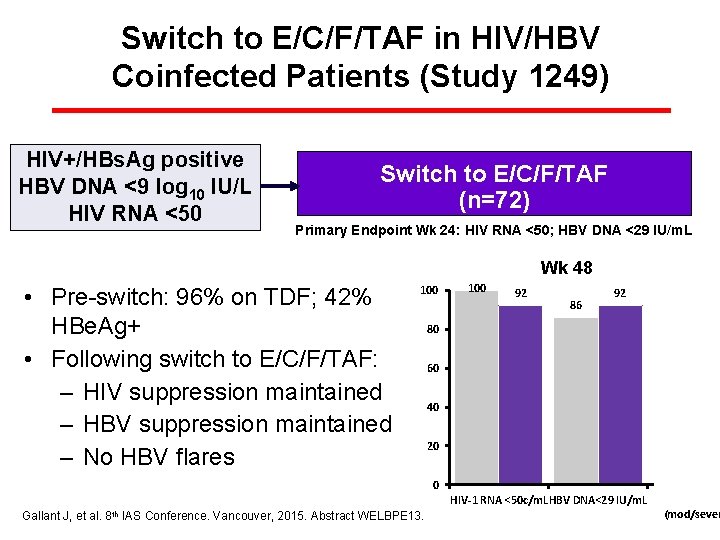
Switch to E/C/F/TAF in HIV/HBV Coinfected Patients (Study 1249) HIV+/HBs. Ag positive HBV DNA <9 log 10 IU/L HIV RNA <50 Switch to E/C/F/TAF (n=72) (n=242) Primary Endpoint Wk 24: HIV RNA <50; HBV DNA <29 IU/m. L Wk 48 • Pre-switch: 96% on TDF; 42% HBe. Ag+ • Following switch to E/C/F/TAF: – HIV suppression maintained – HBV suppression maintained – No HBV flares 100 92 86 92 80 62 60 58 40 20 0 HIV-1 RNA <50 c/m. LHBV DNA<29 IU/m. L Gallant J, et al. 8 th IAS Conference. Vancouver, 2015. Abstract WELBPE 13. Fibrosis (mod/sever

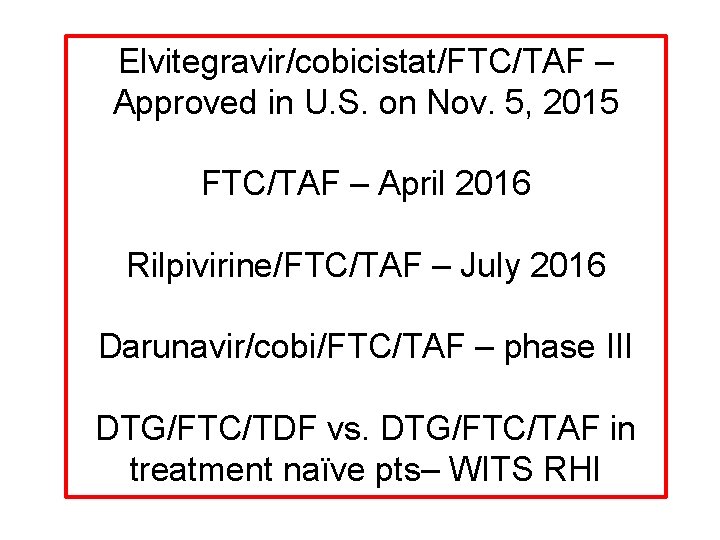
Elvitegravir/cobicistat/FTC/TAF – Approved in U. S. on Nov. 5, 2015 FTC/TAF – April 2016 Rilpivirine/FTC/TAF – July 2016 Darunavir/cobi/FTC/TAF – phase III DTG/FTC/TDF vs. DTG/FTC/TAF in treatment naïve pts– WITS RHI

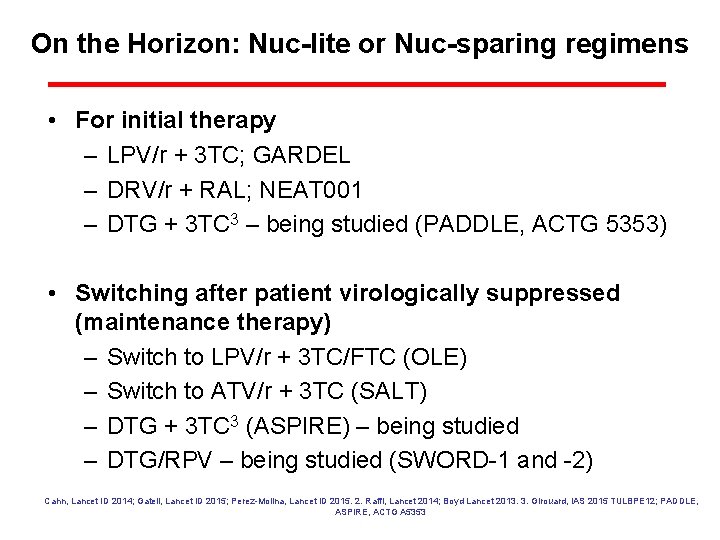
On the Horizon: Nuc-lite or Nuc-sparing regimens • For initial therapy – LPV/r + 3 TC; GARDEL – DRV/r + RAL; NEAT 001 – DTG + 3 TC 3 – being studied (PADDLE, ACTG 5353) • Switching after patient virologically suppressed (maintenance therapy) – Switch to LPV/r + 3 TC/FTC (OLE) – Switch to ATV/r + 3 TC (SALT) – DTG + 3 TC 3 (ASPIRE) – being studied – DTG/RPV – being studied (SWORD-1 and -2) Cahn, Lancet ID 2014; Gatell, Lancet ID 2015; Perez-Molina, Lancet ID 2015. 2. Raffi, Lancet 2014; Boyd Lancet 2013. 3. Girouard, IAS 2015 TULBPE 12; PADDLE, ASPIRE, ACTG A 5353

ART: Long-acting Agents • Long-acting agents: cabotegravir, LArilpivirine, broadlyneutralizing antibodies • Cabotegravir (CBG) and rilpivirine (RPV) available in long-acting nanosuspension formulations which have half-lives of months Latte-2 At wk 32, VL <50 in 95% of q 8 wk arm, 94% of q 4 wk arm, 91% of oral arm. Only 1 pt in an injection arm had virologic failure: no resistance. Spreen, JAIDS 2014; Margolis CROI 2015 #554 LB; Jackson, Clin Pharmacol Ther 2014; Caskey, Nature 2015, Viiv press release, Nov 2015

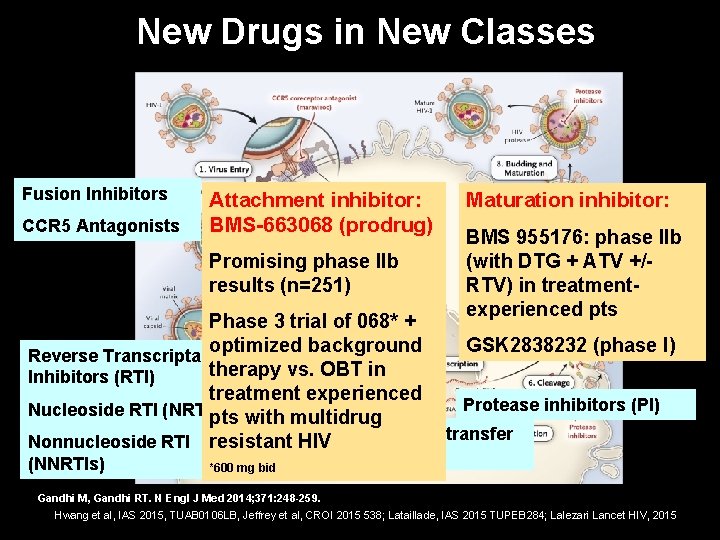
New Drugs in New Classes Fusion Inhibitors CCR 5 Antagonists Reverse Transcriptase Inhibitors (RTI) Protease inhibitors (PI) Nucleoside RTI (NRTIs) Nonnucleoside RTI (NNRTIs) Integrase strand transfer inhibitors (INSTI) Gandhi M, Gandhi RT. N Engl J Med 2014; 371: 248 -259.

New Drugs in New Classes Fusion Inhibitors CCR 5 Antagonists Attachment inhibitor: BMS-663068 (prodrug) Promising phase IIb results (n=251) Maturation inhibitor: BMS 955176: phase IIb (with DTG + ATV +/RTV) in treatmentexperienced pts Phase 3 trial of 068* + GSK 2838232 (phase I) optimized background Reverse Transcriptase therapy vs. OBT in Inhibitors (RTI) treatment experienced Protease inhibitors (PI) Nucleoside RTI (NRTIs) pts with multidrug Integrase strand transfer Nonnucleoside RTI resistant HIV (NNRTIs) *600 mg bid inhibitors (INSTI) Gandhi M, Gandhi RT. N Engl J Med 2014; 371: 248 -259. Hwang et al, IAS 2015, TUAB 0106 LB, Jeffrey et al, CROI 2015 538; Lataillade, IAS 2015 TUPEB 284; Lalezari Lancet HIV, 2015

Challenges and Opportunities • Ending the epidemic with ART? --90/90/90 • Cure?

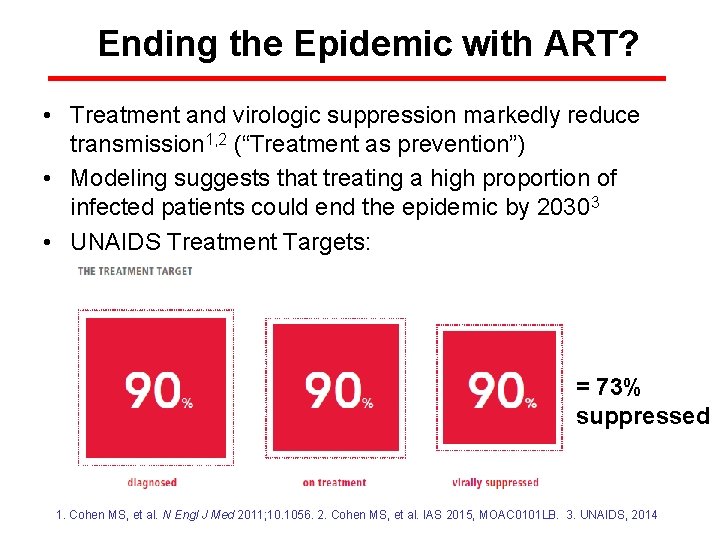
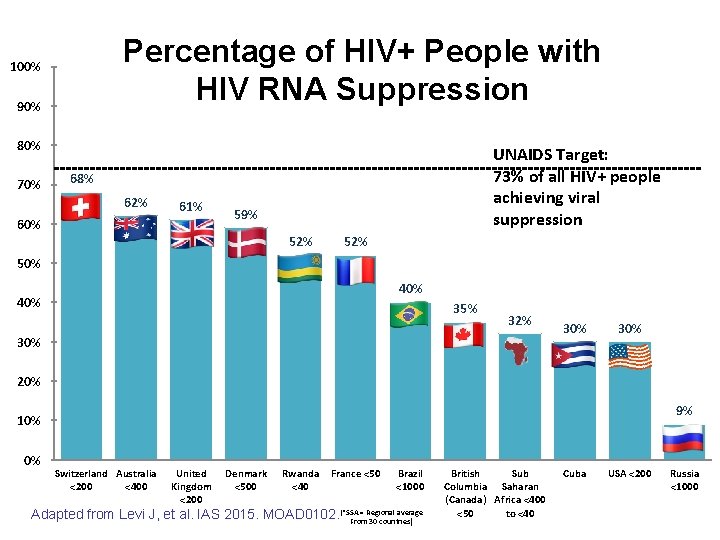
Ending the Epidemic with ART? • Treatment and virologic suppression markedly reduce transmission 1, 2 (“Treatment as prevention”) • Modeling suggests that treating a high proportion of infected patients could end the epidemic by 20303 • UNAIDS Treatment Targets: = 73% suppressed 1. Cohen MS, et al. N Engl J Med 2011; 10. 1056. 2. Cohen MS, et al. IAS 2015, MOAC 0101 LB. 3. UNAIDS, 2014

In which country is the highest proportion of HIV-infected patients virologically suppressed? A. B. C. D. E. U. S. Russia Britain Rwanda Switzerland

Percentage of HIV+ People with HIV RNA Suppression 100% 90% 80% 70% UNAIDS Target: 73% of all HIV+ people achieving viral suppression 68% 62% 61% 60% 59% 52% 50% 40% 35% 32% 30% 30% 20% 9% 10% 0% Switzerland Australia <200 <400 United Kingdom <200 Denmark <500 Rwanda <40 France <50 Brazil <1000 = Regional average Adapted from Levi J, et al. IAS 2015. MOAD 0102. (*SSA From 30 countries) British Sub Columbia Saharan (Canada) Africa <400 <50 to <40 Cuba USA <200 Russia <1000

Case • 55 yo M with HIV infection • “If I take medicines, will I be cured? ” • Only 1 known case of HIV cure: – HIV+ man with leukemia who underwent allogeneic stem cell transplant with a CCRdelta 32 homozygous bone marrow – Following the transplant, he stopped ART and has remained without any evidence for infection

Why Should We Try to Cure HIV? • Although life expectancy has improved, HIV+ patients, especially if diagnosed late, don’t live as long as HIV-negative people -- perhaps because of persistent inflammation in HIV+ patients • Cost, side effects, and impact on quality of life-long ART • Need to maintain high level of adherence to ART to prevent drug-resistant virus • Stigma, discrimination, fear of transmission, isolation Adapted from slide by Joe Eron, MD

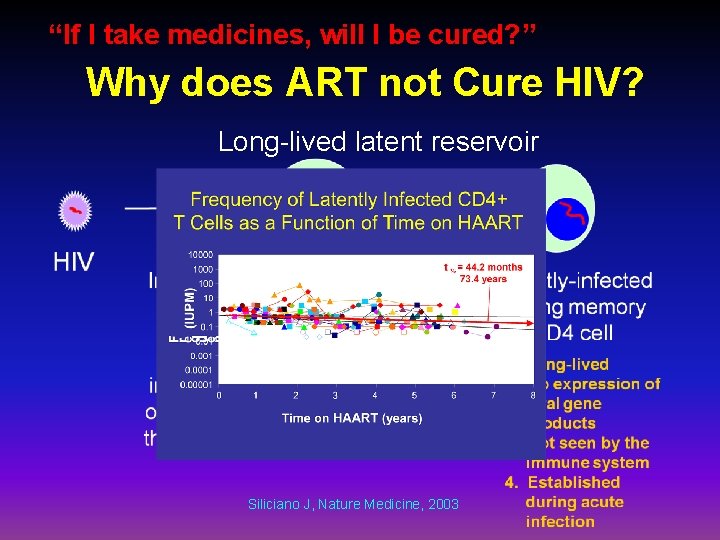
“If I take medicines, will I be cured? ” Why does ART not Cure HIV? Long-lived latent reservoir Siliciano J, Nature Medicine, 2003

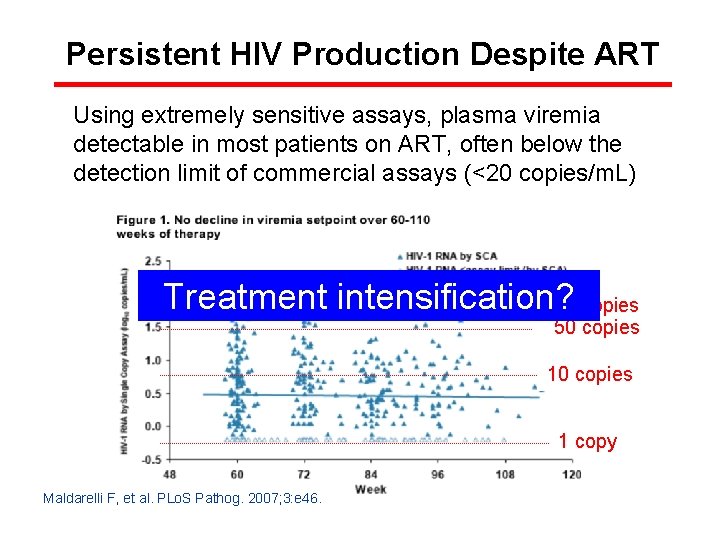
Persistent HIV Production Despite ART Using extremely sensitive assays, plasma viremia detectable in most patients on ART, often below the detection limit of commercial assays (<20 copies/m. L) Treatment intensification? 100 copies 50 copies 1 copy Maldarelli F, et al. PLo. S Pathog. 2007; 3: e 46.

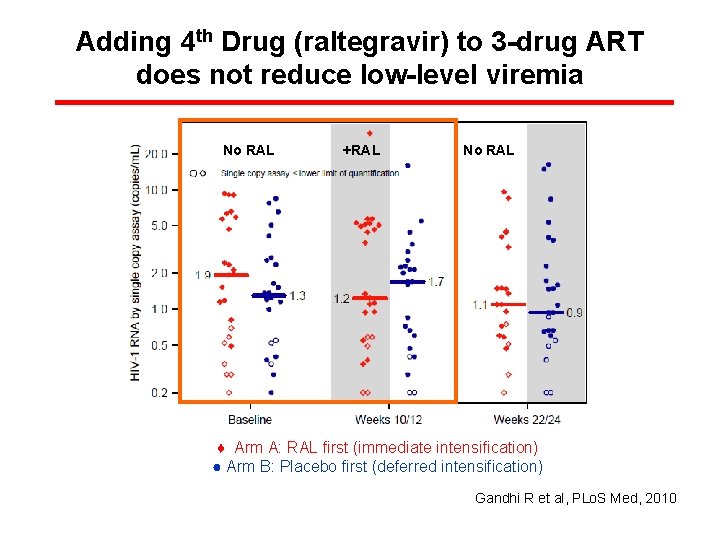
Adding 4 th Drug (raltegravir) to 3 -drug ART does not reduce low-level viremia No RAL +RAL No RAL ♦ Arm A: RAL first (immediate intensification) ● Arm B: Placebo first (deferred intensification) Gandhi R et al, PLo. S Med, 2010

Can we cure HIV? “It's tough to make predictions, especially about the future”

Can we cure HIV? “ART-Plus” • HIV cure remains an aspirational goal • Clinical trials are testing ways to: • Reverse latency • Enhance killing of infected cells • Make cells impervious to infection • Combination therapy!! • Increased knowledge of mechanisms of HIV persistence needed • Given safety of current ART and uncertainties regarding risks of novel interventions, cure studies must adhere to highest scientific and ethical standards

World AIDS Day, 2013: . . . the United States should be at the forefront of new discoveries into how to put HIV into long-term remission without requiring lifelong therapies -- or, better yet, to eliminate it completely. Patient: One day I’d love to say, “I used to have HIV. ”
 Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Immediate update and deferred update in dbms
Immediate update and deferred update in dbms Fibrinolytic checklist time goal stroke
Fibrinolytic checklist time goal stroke Ttkg formula
Ttkg formula Official disability guidelines
Official disability guidelines Ppcd ascites
Ppcd ascites Allergic crease adalah
Allergic crease adalah Allergic rhinitis treatment guidelines
Allergic rhinitis treatment guidelines Ich treatment guidelines
Ich treatment guidelines Hepatic encephalopathy staging
Hepatic encephalopathy staging What is odg guidelines
What is odg guidelines Glasser modeli
Glasser modeli Y connected generator
Y connected generator Difference between phase voltage and line voltage
Difference between phase voltage and line voltage N=nc exp(-eg/2kt)
N=nc exp(-eg/2kt) Lesson 4 three-phase motors
Lesson 4 three-phase motors Drift current and diffusion current in semiconductor
Drift current and diffusion current in semiconductor Drift current and diffusion current
Drift current and diffusion current In a triangle connected source feeding a y connected load
In a triangle connected source feeding a y connected load Slideplayer
Slideplayer Diffusion current formula
Diffusion current formula Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Bioness bits cost
Bioness bits cost Humanistic therapies aim to boost
Humanistic therapies aim to boost The constant current region of a fet lies between
The constant current region of a fet lies between In generators the welding current is produced on the ____.
In generators the welding current is produced on the ____. Hazard based safety engineering
Hazard based safety engineering Mesh current method with current source
Mesh current method with current source Develop and update food and beverage knowledge
Develop and update food and beverage knowledge Sql insert update delete query
Sql insert update delete query Data redundancy and update anomalies
Data redundancy and update anomalies Zechariah
Zechariah Zechariah 2:8-9
Zechariah 2:8-9 University community plan update
University community plan update Temporary update problem in dbms
Temporary update problem in dbms Sab abdate.com
Sab abdate.com Routing area update
Routing area update Update data sisdmk
Update data sisdmk Gtcs professional update examples
Gtcs professional update examples Position update formula
Position update formula Position update formula
Position update formula Fiberhome firmware download
Fiberhome firmware download Move update compliance
Move update compliance Mdh situation update
Mdh situation update Iqcs login
Iqcs login Cucm native call queuing
Cucm native call queuing 811 locate request
811 locate request Location update procedure
Location update procedure Chrome update
Chrome update Amno region
Amno region