UPCOMING CHANGES TO INVITRO DIAGNOSTICS IVDs AND LABORATORY
UPCOMING CHANGES TO IN-VITRO DIAGNOSTICS (IVDs) AND LABORATORY DEVELOPED TESTS (LDTs) REGULATIONS Moj Eram, Ph. D November 5, 2015

IN-VITRO DIAGNOSTIC (IVD) Overview § § § IVD definition LDT definition Comparison of LVD and LDT EU proposed changes to IVD Regulation US draft guidance and proposed changes to LDT regulation Summary

IN-VITRO DIAGNOSTIC (IVD) § In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body. § Types of IVDs – Intended to be used: § In the laboratory § By health professionals at the point of care (i. e. hospitals, Laboratories) § By lay –person (self testing) § Regulatory authority § Medical devices § Biological products § IVDs - subject to the Clinical Laboratory Improvement Amendments (CLIA '88) of 1988.
LABORATORY DEVELOPED TEST (LDT) • A laboratory Developed Tests (LDTs) are in vitro diagnostic tests that are developed, validated and used within a single laboratory (CLIA Certified) • Patients are treated in the facility where test is developed • It can not be used to specify a diagnosis; can state correlation of outcome of a test to a likely outcome • Does not require FDA approval or clearance • Labs not tests are regulated under CLIA
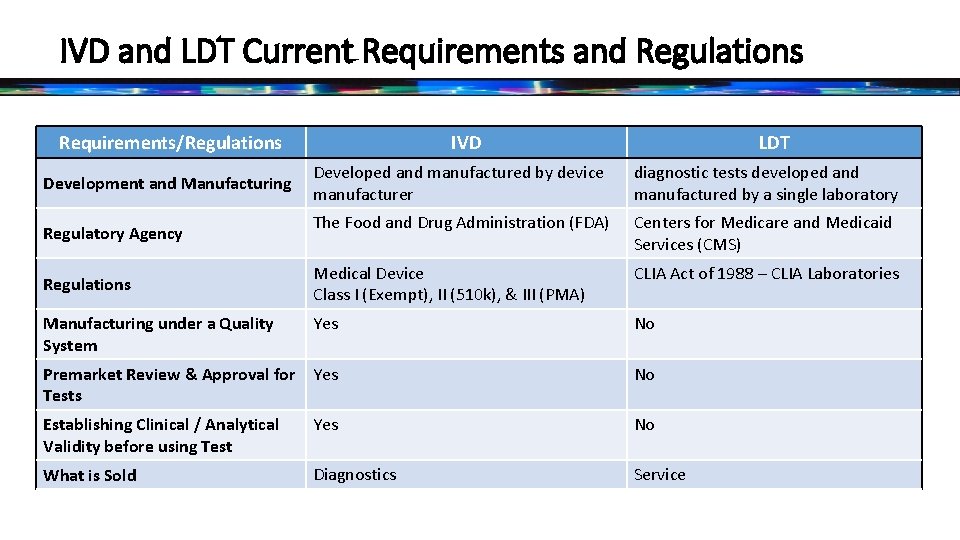
IVD and LDT Current Requirements and Regulations Requirements/Regulations IVD LDT Developed and manufactured by device manufacturer diagnostic tests developed and manufactured by a single laboratory The Food and Drug Administration (FDA) Centers for Medicare and Medicaid Services (CMS) Medical Device Class I (Exempt), II (510 k), & III (PMA) CLIA Act of 1988 – CLIA Laboratories Yes No Premarket Review & Approval for Yes Tests No Establishing Clinical / Analytical Validity before using Test Yes No What is Sold Diagnostics Service Development and Manufacturing Regulatory Agency Regulations Manufacturing under a Quality System
EU – PROPOSED CHANGES TO IVD REGULATIONS § The “New Approach” is prompted by: § Rapid advances in medical device technologies and health care development § Recent finding of safety issues/concerns. § Increase focus on preventive strategies, early diagnostics, need for more self monitoring tests and cost effectiveness § Globalization – need for harmonized regulations § Public expectations § IVD market projection of $22 Bn in the EU by 2018, nearly 80% of IVD devices could be subjected to notified body approval, from the currently 20%. § New regulations expected to pass in 2015 – 2016 § Require manufacturers 5 years to become compliant

EU - UPCOMING PROPOSED CHANGES TO IVD REGULATIONS § Overview of proposed changes: § Extension of regulatory scope – to include high risk IVD manufactured and used within single health institution (LDT) § classification structure for IVD – risk based classification: § 4 classes - A (low risk), B, C, D (Highest risk) § Changes in notified body unannounced audits, assessment and oversight § Increased conformity assessment § Clinical evidence - will require for all high risk devices (C & D), clinical literature will not be sufficient § Post market surveillance § Implementation of unique device identification requirements § “Qualified person” for regulatory compliance within manufacturer – assess the conformity of the product § Expanded registration database of devices
EU – IMPACT OF IVD REGULATION CHANGES § Anticipated impact on IVD manufacturers: § Broader applicability of regulatory requirements § More requirements for device safety and performance § Increased unannounced inspection (audits) of suppliers and more § Increased post market follow up § Competent authorities performing risk assessment – more scrutiny § Greater provisions for transparency and traceability § Increase time to market § Increased capital need
US - LDT REGULATIONS AND RECENT CHANGES § Currently, unlike IVDs, LDTs do not require FDA approval or clearance § FDA proposed regulatory changes due to: § Increased risk associated with LDT’s as IVDs § Inadequate controls and monitoring of tests § LDT are used more broadly outside of the manufactured laboratory § Due to increased complexity of the test § Criticality in clinical decision making in the context of personalized medicine (i. e. genetic testing)
US - LDT REGULATIONS AND RECENT CHANGES § Draft guidance - Framework for Regulatory Oversight of LDT - issued October 3, 2014 § Risk-based regulatory framework phase over 9 years § All requirements are begin to take effect 6 months after the guidance is finalized § low risk LDT (Class I), traditional LDT, forensic, transplantation, and LDTs intended for rare diseases and unmet needs are exempt form all regulations except for: § Device registration § Device listing § Medical Device Reporting (MDR) § Highest risk LDTs will enforced first – same intended use as cleared/approved IVDs § Moderate and high risk LDTs are subject to more rigorous regulatory requirements § Premarket and post market review for all NEW LDTs

SUMMARY § Change is coming … Plan accordingly and get ahead and stay ahead of regulations § Have a proactive strategy to understand prepare for changes to minimize transition time and reduce risk § For IVD regulation changes in the EU: § Begin classification of your device and plan for new certification requirements § Conduct GAP analysis to check requirements and QMR § Implement Quality Management System (QMS) updates § Understand the role of Notified bodies and plan for increased approval time (budget accordingly) § Give time for implementation/transition § For LDTs regulation changes in the US: § Understand device classification of LDT § Understand the quality and regulatory requirements (GAP Analysis) § Understand the market

THANK YOU Moj Eram, Ph. D Moj. eram@gmail. com
- Slides: 12