Unstable Nuclei Radioactive Decay Radioactivity n n Nucleus

Unstable Nuclei & Radioactive Decay

Radioactivity n n Nucleus of an element spontaneously emits subatomic particles & electromagnetic waves. Nucleus changes into a different element when it does this. Original nucleus is called “unstable. ” Process is called “decay” or “transmutation. ”


Rutherford & Radioactivity n n 1898 – Rutherford began experiments with radioactivity. 1899 – discovered alpha and beta “rays” from uranium.
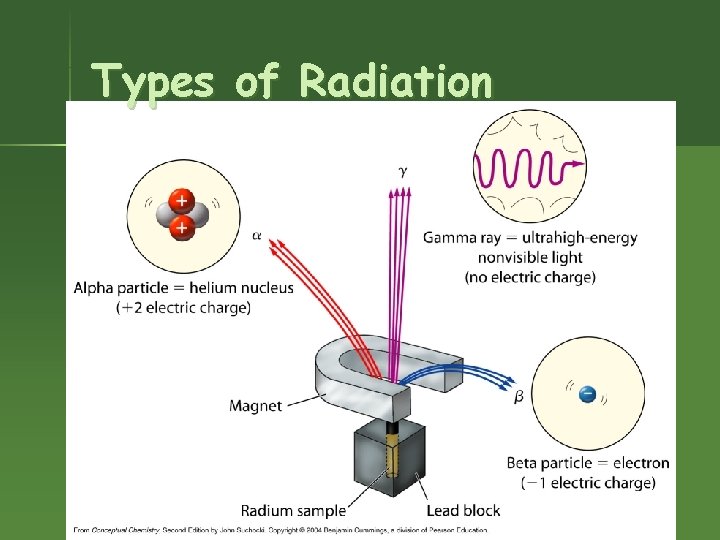
Types of Radiation
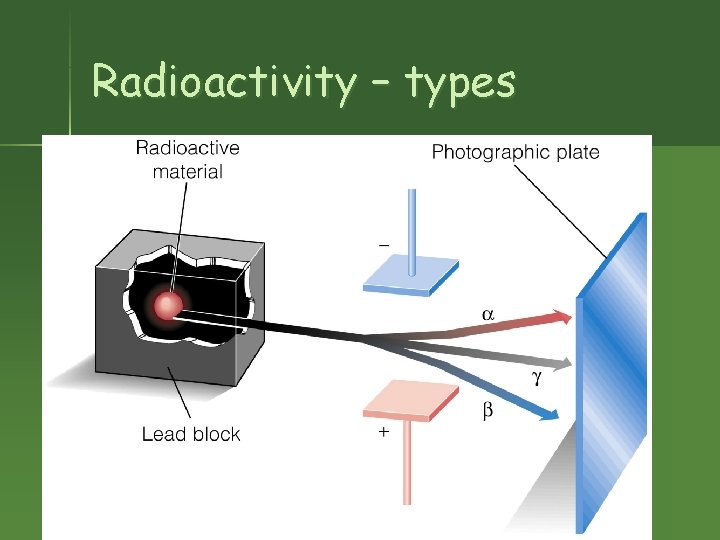
Radioactivity – types
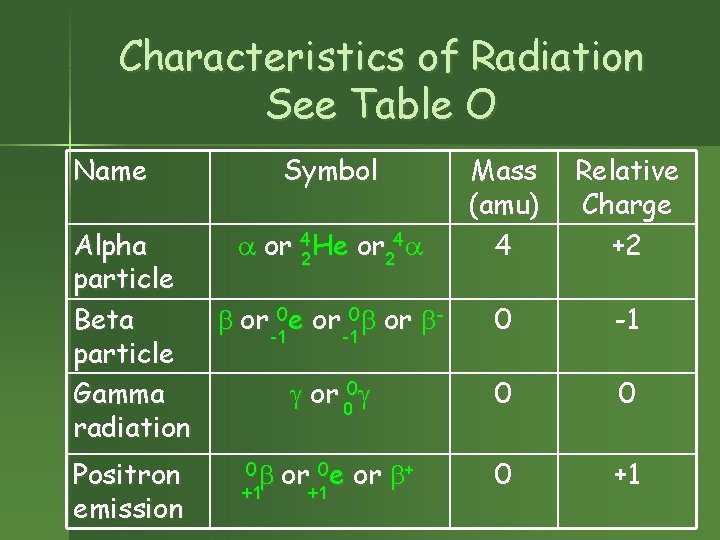
Characteristics of Radiation See Table O Name Symbol Alpha or 42 He or 24 particle Beta or 0 e or 0 or -1 -1 particle Gamma or 00 radiation Positron emission 0 +1 or 0 e or + +1 +1 Mass (amu) 4 Relative Charge +2 0 -1 0 0 0 +1
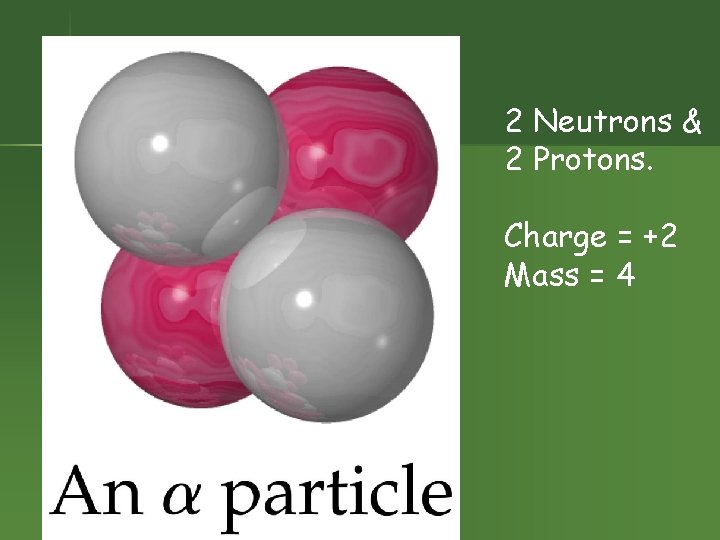
2 Neutrons & 2 Protons. Charge = +2 Mass = 4

Beta Particle – fast moving electron.
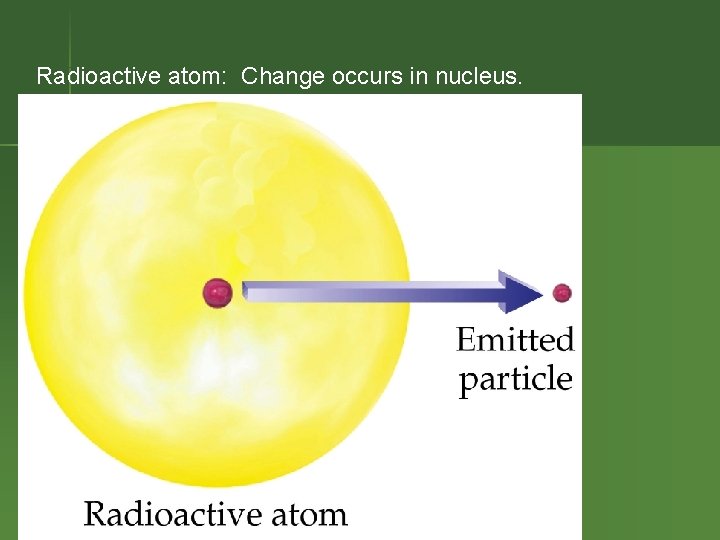
Radioactive atom: Change occurs in nucleus.
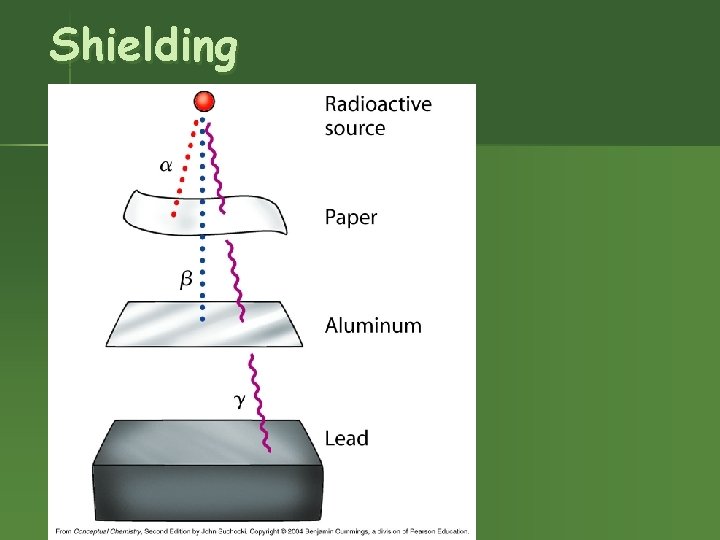
Shielding

Can we predict exactly when an atom will decay? n n NO! For large #’s of atoms, we CAN predict how many will decay on average in a given amount of time.
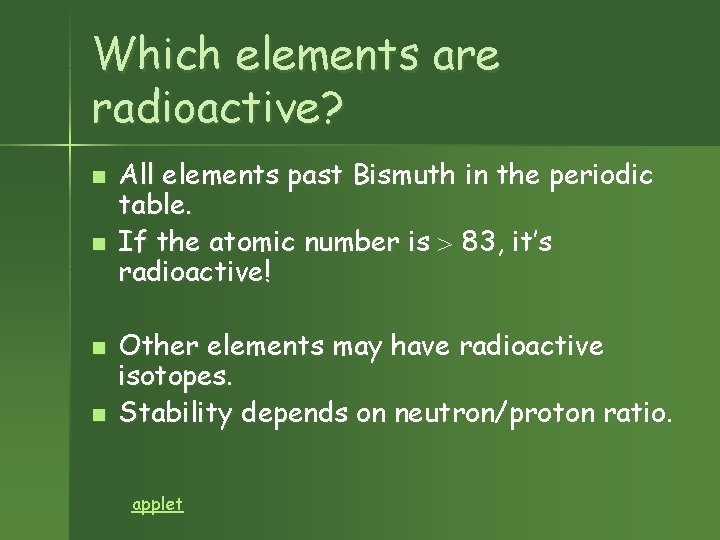
Which elements are radioactive? n n All elements past Bismuth in the periodic table. If the atomic number is 83, it’s radioactive! Other elements may have radioactive isotopes. Stability depends on neutron/proton ratio. applet

What’s Going on in the Nucleus? Electrostatic repulsions between protons. Want to fly apart. n But protons & neutrons all attracted to each other by nuclear strong force. n n So having neutrons helps hold a nucleus together.
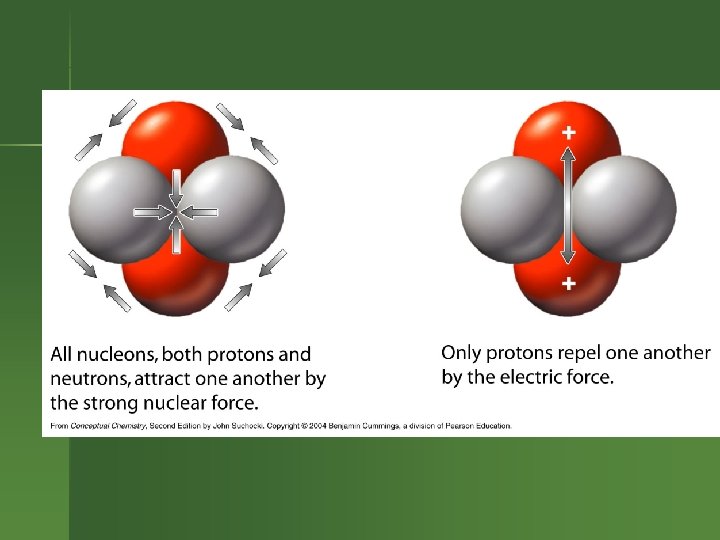

Strong Force Works best if the nucleus isn’t too large. n As the nucleus gets larger, need to have more neutrons to help counteract the electrostatic repulsion between the protons. n Eventually, the nucleus is too large to be stable. n
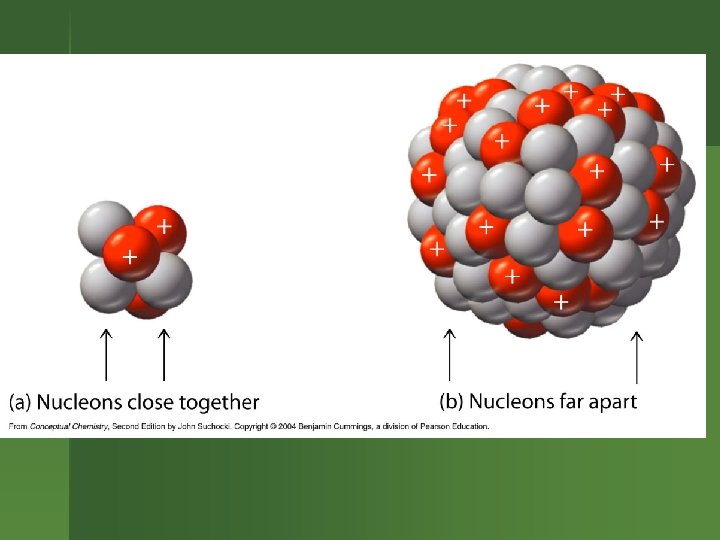
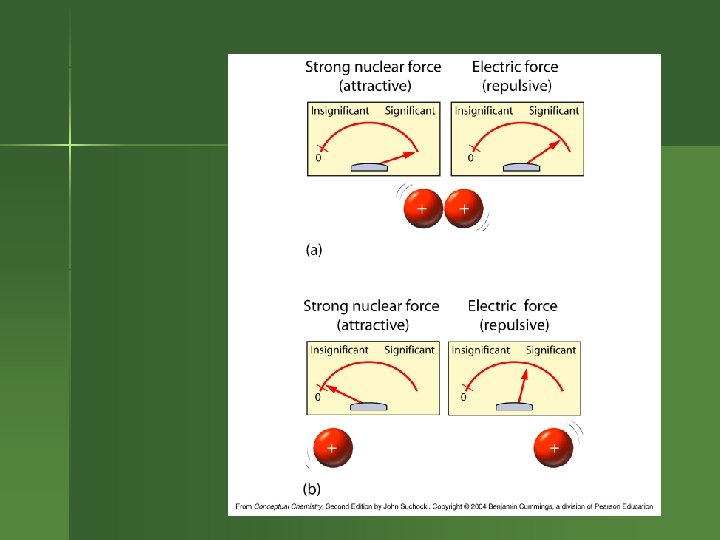

Balancing Act n n Balance exists between electrostatic repulsive force & nuclear strong force. Certain #’s of protons & neutrons make a stable nucleus. Other #’s of protons & neutrons are unstable. So the atom decays.
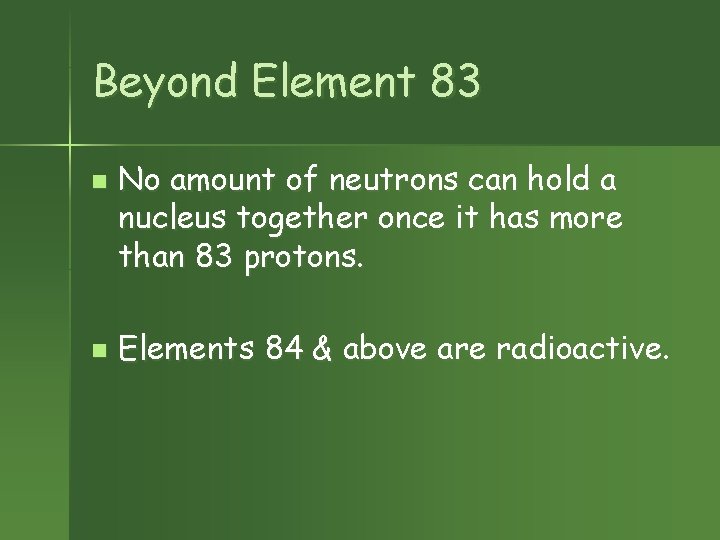
Beyond Element 83 n n No amount of neutrons can hold a nucleus together once it has more than 83 protons. Elements 84 & above are radioactive.
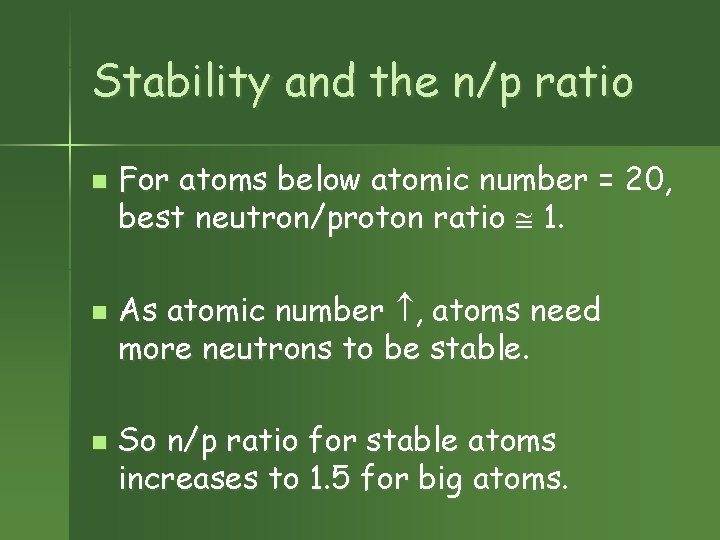
Stability and the n/p ratio n n n For atoms below atomic number = 20, best neutron/proton ratio 1. As atomic number , atoms need more neutrons to be stable. So n/p ratio for stable atoms increases to 1. 5 for big atoms.
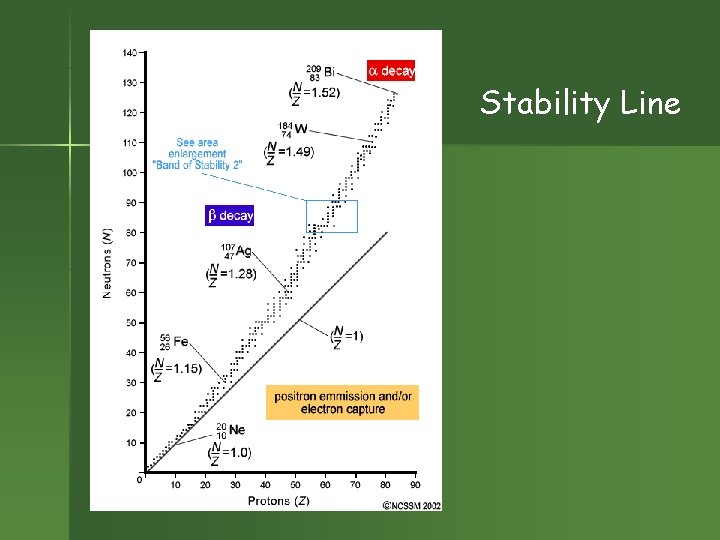
Stability Line
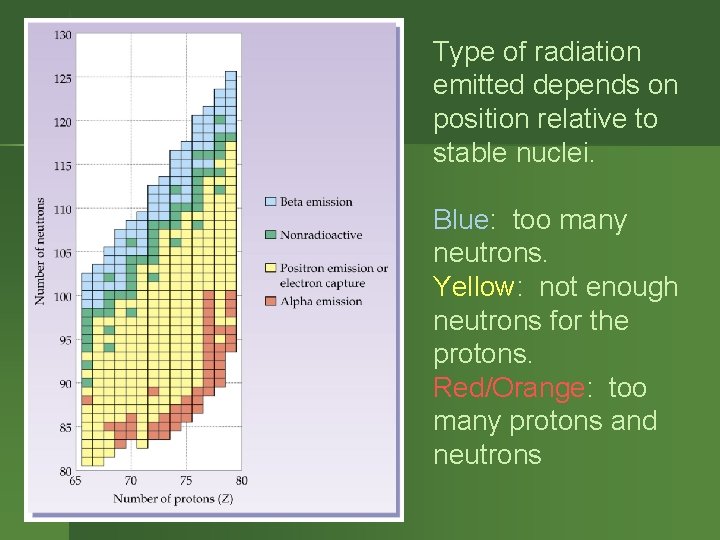
Type of radiation emitted depends on position relative to stable nuclei. Blue: too many neutrons. Yellow: not enough neutrons for the protons. Red/Orange: too many protons and neutrons

Natural Radioactivity – Unstable Nuclei Emit Radiation Spontaneous nuclear change to attain good n/p ratio (high stability, low energy state). n Form a new kind of atom. n n Each isotope or nuclide decays in a certain manner to get a better n/p ratio. The decay mode is named for the particle emitted. See Table N.
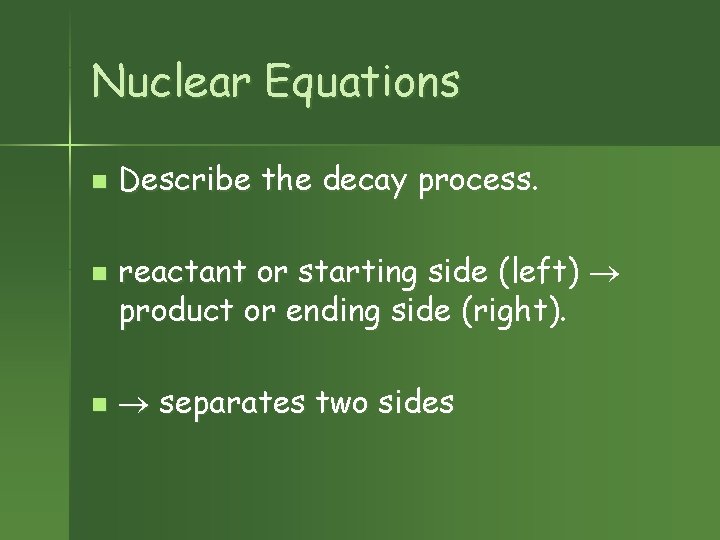
Nuclear Equations n n n Describe the decay process. reactant or starting side (left) product or ending side (right). separates two sides
Nuclear vs. Chemical n How is a nuclear change different from a chemical change?

NUCLEAR n n n Involve a change in an atom’s nucleus. Radioactive atoms spontaneously emit radiation and change into other kinds of atoms. Nuclear reactions involve 1, 000 X more energy than ordinary chemical rxns. CHEMICAL n n n Involve changes in the outermost electrons. 1 or more substances changed into new substances. Atoms are rearranged, but their identities do not change.
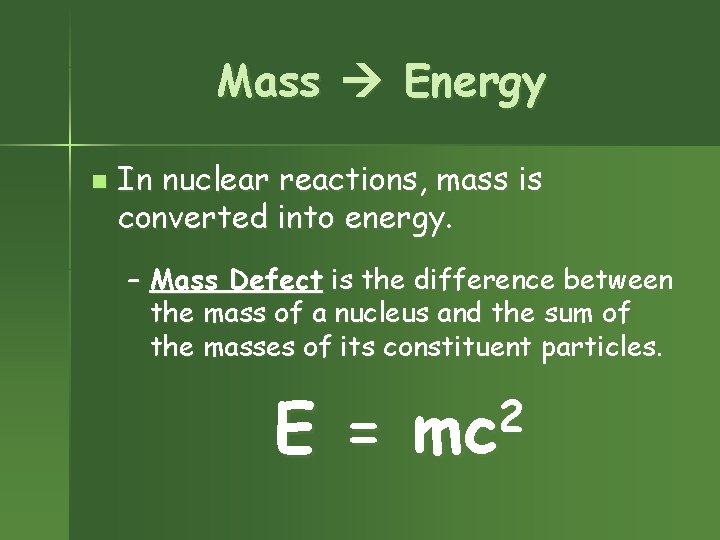
Mass Energy n In nuclear reactions, mass is converted into energy. – Mass Defect is the difference between the mass of a nucleus and the sum of the masses of its constituent particles. E = 2 mc
Binding Energy n n Energy released when a nucleus is formed from its constituent particles. It is a measure of the stability of an atom formed: – The higher the binding energy the more stable the nucleus. – The lightest and heaviest elements are the most unstable (low BE) – Intermediate elements are the most stable (highest BE).
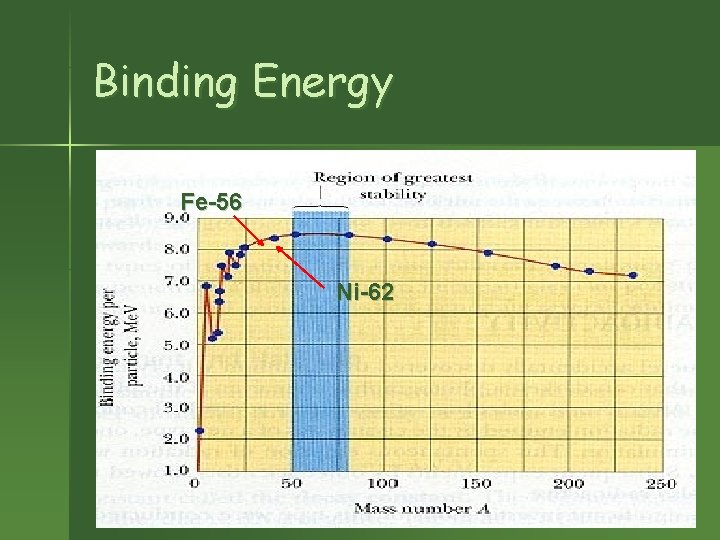
Binding Energy Fe-56 Ni-62
- Slides: 30