Unresolved procedural regulatory and statistical issues in assessing








- Slides: 24

Unresolved procedural, regulatory and statistical issues in assessing human QT prolongation and performing the ‘thorough QT study’ Borje Darpo MD Ph. D, FESC Associate Professor in Cardiology Pharmaceutical Consultant borje. darpo@telia. com QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

Objective of presentation To outline some areas within clinical QT assessment, which I believe need more studies and publicly shared data to allow for definitive recommendations ‘More than one road lead to Rome – until someone builds a motorway’ Hieronymus ‘Filibuster’ Hippocampus A. D. -07 QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

Some unresolved issues – not necessarily the most important ones Thorough QT study 1. Baseline assessment 2. Heart rate correction algorithm for drugs without or with a small inherent effect on the heart rate 3. The role of the positive control 4. Which effect of the positive control establishes assay sensitivity? 5. How should emerging new techniques be validated? QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

1. Baseline in the TQT study Background: Adjustment for baseline measurements is important for reducing the influence of inter-subject differences and general, as well as study-specific, diurnal effects such as those due to food Currently ‘recommended’ (code for ‘recently requested by the FDA’s IRT’): Cross-over studies: Predose baseline immediately before dosing on treatment day in each period Parallel studies: One full, ‘time-matched’ baseline on the day before dosing in each treatment group QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

Baseline in parallel TQT studies 1. Pre-dose baseline: Immediately before dosing on Day 1 in each Tx group. 2. Time-matched baseline (‘recommended’): Recorded at same time-points on Day -1 as after dosing on Day 1. 3. Time-averaged: Recorded as above, but baseline derived from average of Day -1 values QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

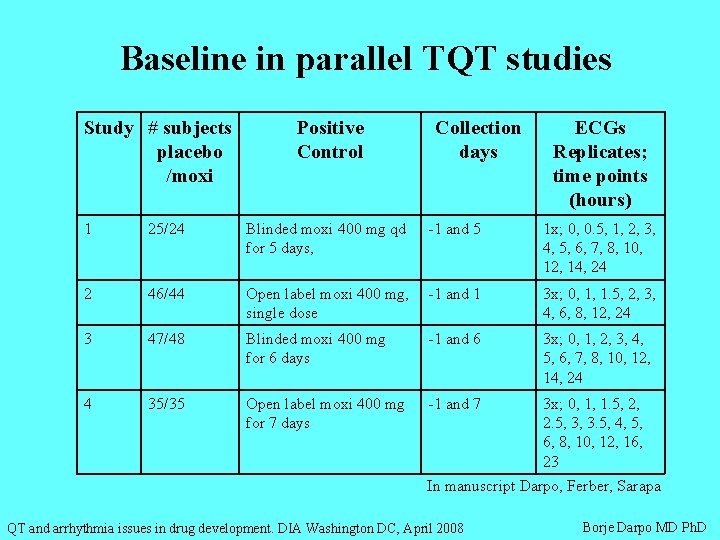
Baseline in parallel TQT studies Study # subjects placebo /moxi Positive Control Collection days ECGs Replicates; time points (hours) 1 25/24 Blinded moxi 400 mg qd for 5 days, -1 and 5 1 x; 0, 0. 5, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 24 2 46/44 Open label moxi 400 mg, single dose -1 and 1 3 x; 0, 1, 1. 5, 2, 3, 4, 6, 8, 12, 24 3 47/48 Blinded moxi 400 mg for 6 days -1 and 6 3 x; 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 24 4 35/35 Open label moxi 400 mg for 7 days -1 and 7 3 x; 0, 1, 1. 5, 2, 2. 5, 3, 3. 5, 4, 5, 6, 8, 10, 12, 16, 23 In manuscript Darpo, Ferber, Sarapa QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

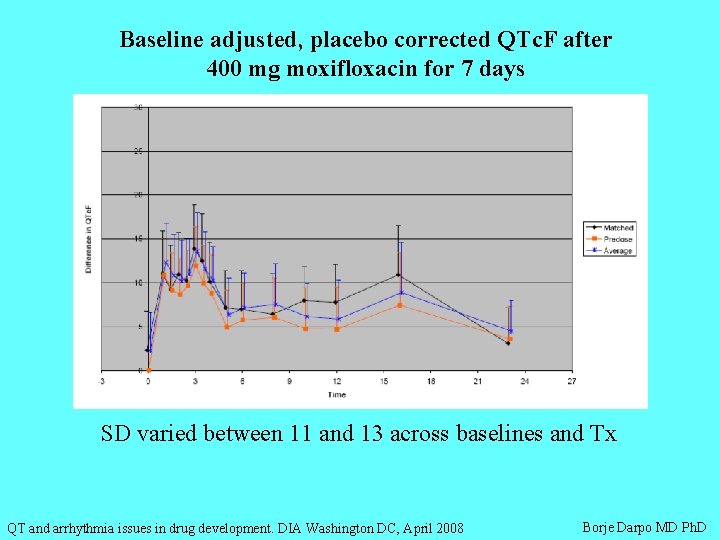
Baseline adjusted, placebo corrected QTc. F after 400 mg moxifloxacin for 7 days SD varied between 11 and 13 across baselines and Tx QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

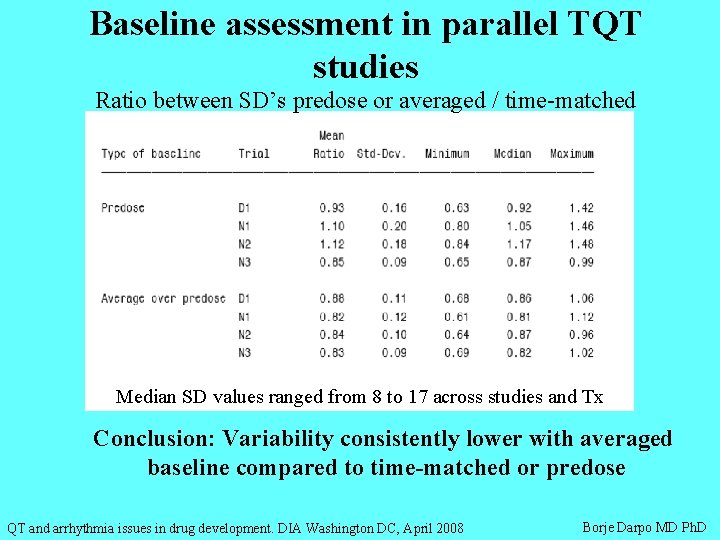
Baseline assessment in parallel TQT studies Ratio between SD’s predose or averaged / time-matched Median SD values ranged from 8 to 17 across studies and Tx Conclusion: Variability consistently lower with averaged baseline compared to time-matched or predose QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

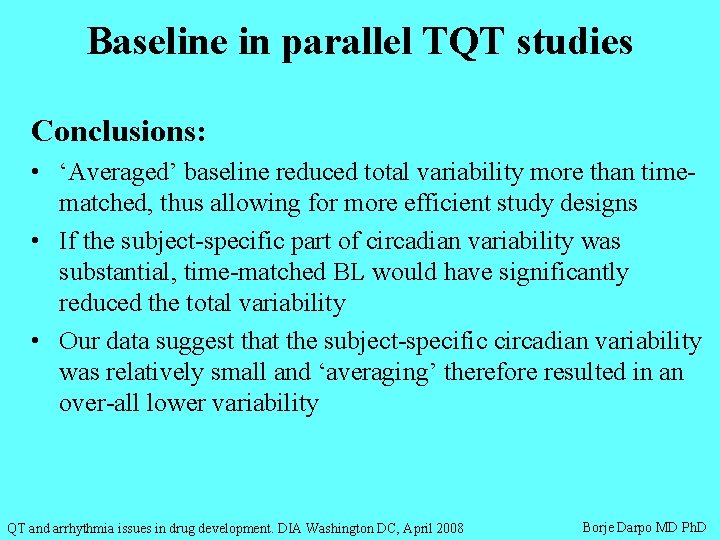
Baseline in parallel TQT studies Conclusions: • ‘Averaged’ baseline reduced total variability more than timematched, thus allowing for more efficient study designs • If the subject-specific part of circadian variability was substantial, time-matched BL would have significantly reduced the total variability • Our data suggest that the subject-specific circadian variability was relatively small and ‘averaging’ therefore resulted in an over-all lower variability QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

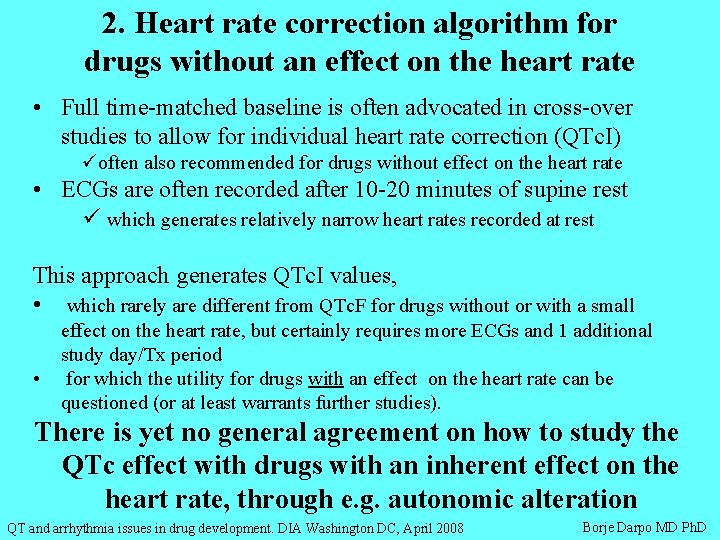
2. Heart rate correction algorithm for drugs without an effect on the heart rate • Full time-matched baseline is often advocated in cross-over studies to allow for individual heart rate correction (QTc. I) üoften also recommended for drugs without effect on the heart rate • ECGs are often recorded after 10 -20 minutes of supine rest ü which generates relatively narrow heart rates recorded at rest This approach generates QTc. I values, • which rarely are different from QTc. F for drugs without or with a small • effect on the heart rate, but certainly requires more ECGs and 1 additional study day/Tx period for which the utility for drugs with an effect on the heart rate can be questioned (or at least warrants further studies). There is yet no general agreement on how to study the QTc effect with drugs with an inherent effect on the heart rate, through e. g. autonomic alteration QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

3. Why are we using a positive control? According to ICH E 14: “The confidence in the ability of the study to detect QT/QTc prolongation can be greatly enhanced by the use of a concurrent positive control group to establish assay sensitivity“ “…, the positive control (whether pharmacological or nonpharmacological) should be well-characterized and consistently produce an effect corresponding to the largest change in the QT/QTc interval that is currently viewed as clinically not important to detect (a mean change of around 5 ms or less)” QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

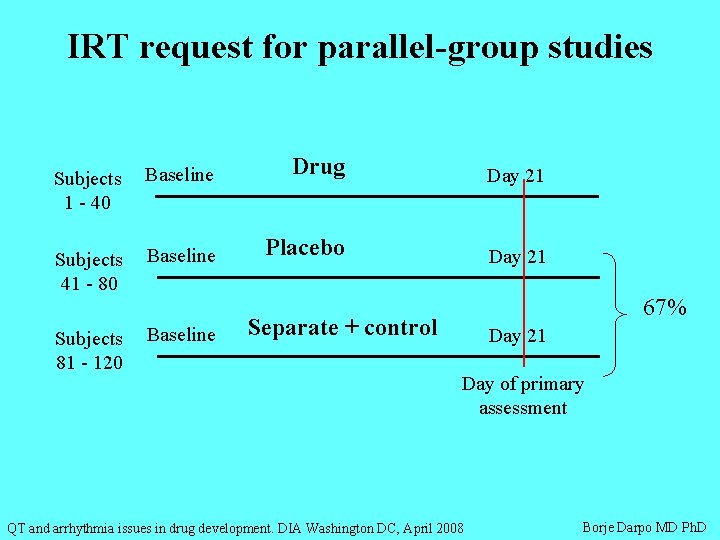
Establishing assay sensitivity • Uncontroversial in X-over studies with short duration in which + control can be included as separate Tx period • Challenging in studies with long duration of Tx or long wash-out (e. g. dose escalation, drug accumulation) • Recently the IRT has requested running the positive control in separate Tx group in parallel TQT studies – which means that 67% of subjects are exposed to either placebo or one dose of moxifloxacin QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

IRT request for parallel-group studies Subjects 1 - 40 Baseline Drug Day 21 Subjects 41 - 80 Baseline Placebo Day 21 Subjects 81 - 120 Baseline 67% Separate + control Day 21 Day of primary assessment QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

Question: Does the gain with separate + control Tx-group justify the added cost and complexity of the study? Could an alternative approach be acceptable? All Tx periods are extended by 1 day and a single-dose of + control/placebo is added on this day Issue: Baseline adjustment and placebo-correction will be within group for + control effect but not for drug effect QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

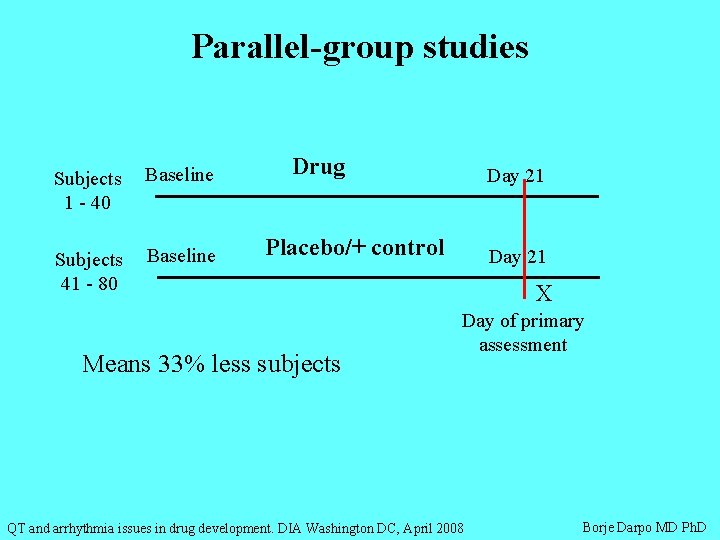
Parallel-group studies Subjects 1 - 40 Baseline Subjects 41 - 80 Baseline Drug Day 21 Placebo/+ control Means 33% less subjects Day 21 X Day of primary assessment QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

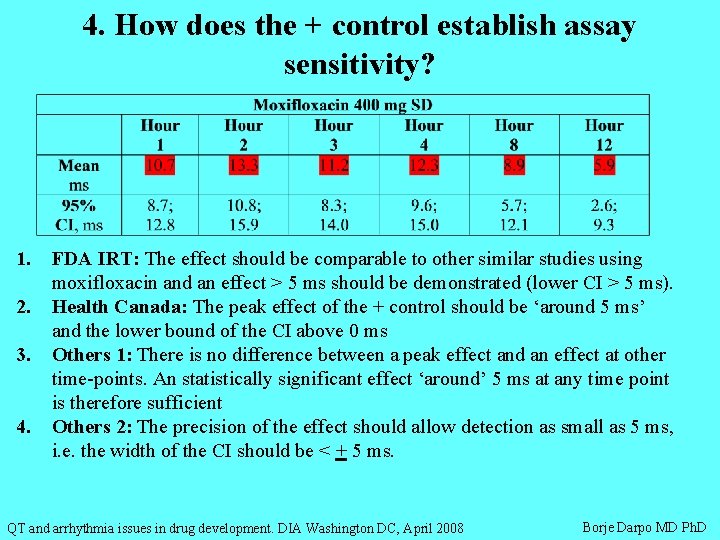
4. How does the + control establish assay sensitivity? 1. 2. 3. 4. FDA IRT: The effect should be comparable to other similar studies using moxifloxacin and an effect > 5 ms should be demonstrated (lower CI > 5 ms). Health Canada: The peak effect of the + control should be ‘around 5 ms’ and the lower bound of the CI above 0 ms Others 1: There is no difference between a peak effect and an effect at other time-points. An statistically significant effect ‘around’ 5 ms at any time point is therefore sufficient Others 2: The precision of the effect should allow detection as small as 5 ms, i. e. the width of the CI should be < + 5 ms. QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

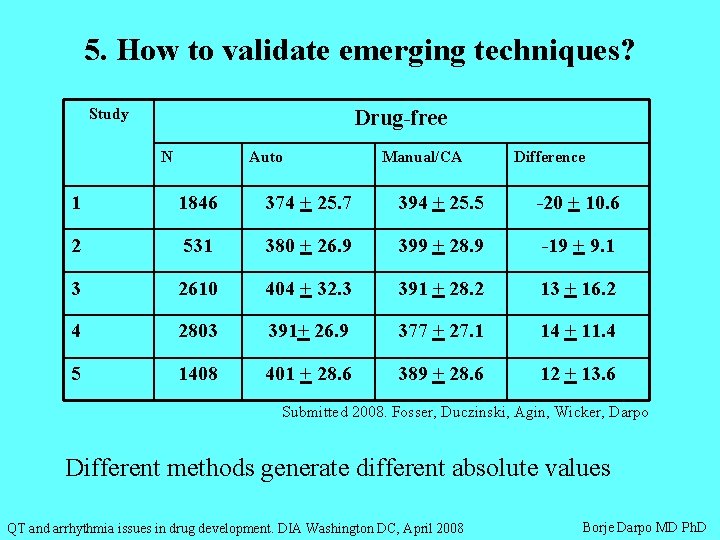
5. How to validate emerging techniques? Study Drug-free N Auto Manual/CA Difference 1 1846 374 + 25. 7 394 + 25. 5 -20 + 10. 6 2 531 380 + 26. 9 399 + 28. 9 -19 + 9. 1 3 2610 404 + 32. 3 391 + 28. 2 13 + 16. 2 4 2803 391+ 26. 9 377 + 27. 1 14 + 11. 4 5 1408 401 + 28. 6 389 + 28. 6 12 + 13. 6 Submitted 2008. Fosser, Duczinski, Agin, Wicker, Darpo Different methods generate different absolute values QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

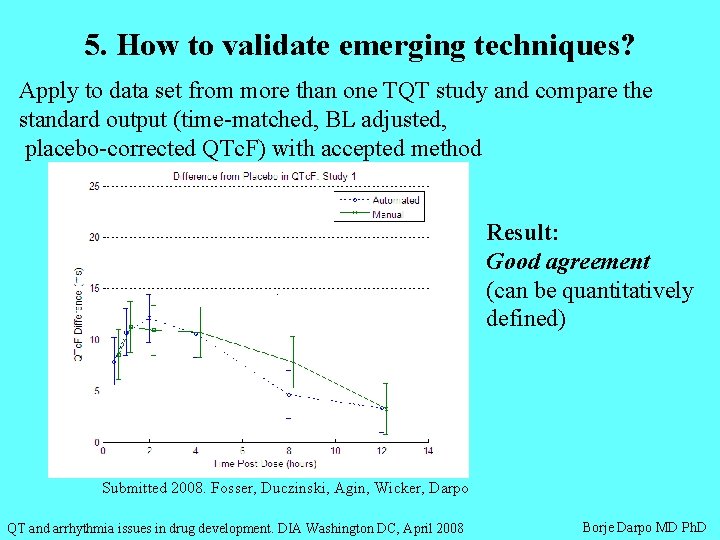
5. How to validate emerging techniques? Apply to data set from more than one TQT study and compare the standard output (time-matched, BL adjusted, placebo-corrected QTc. F) with accepted method Result: Good agreement (can be quantitatively defined) Submitted 2008. Fosser, Duczinski, Agin, Wicker, Darpo QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

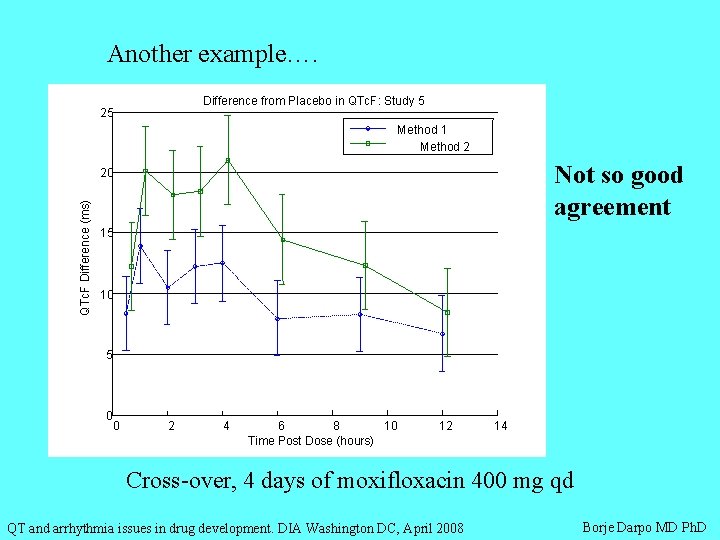
Another example…. Difference from Placebo in QTc. F: Study 5 25 Method 1 Method 2 Not so good agreement QTc. F Difference (ms) 20 15 10 5 0 0 2 4 6 8 10 Time Post Dose (hours) 12 14 Cross-over, 4 days of moxifloxacin 400 mg qd QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

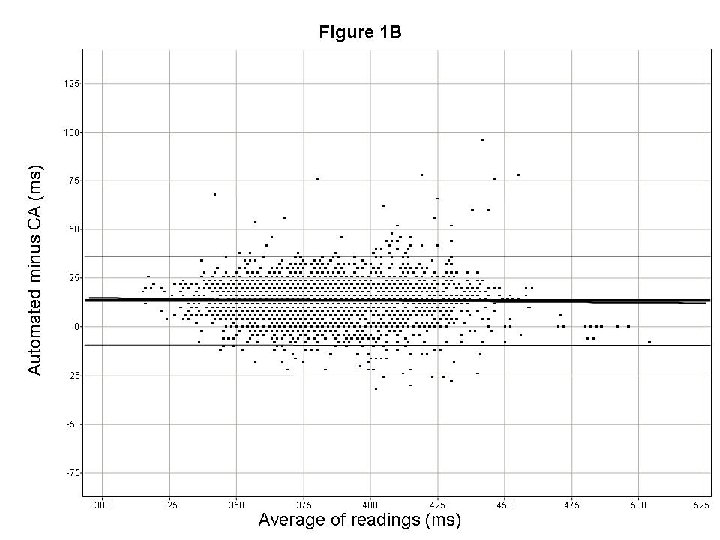
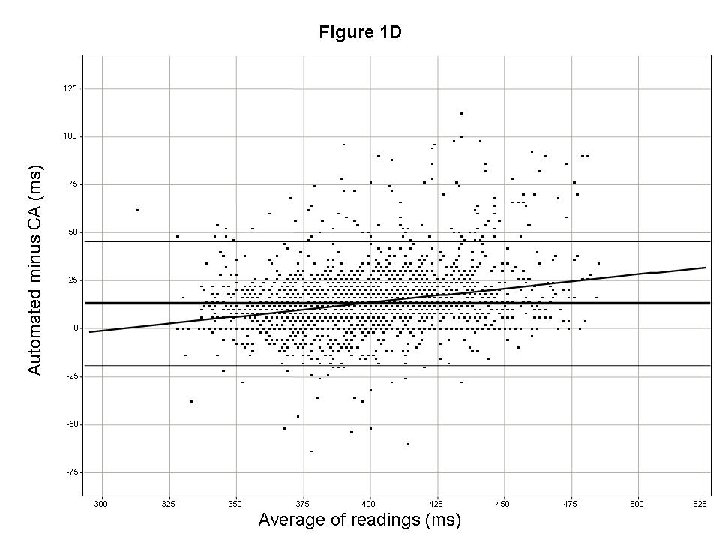
How to validate new techniques? Recommendations • Validate new measurement methods (techniques) on data set from TQT studies – • Look at data several ways, e. g. – – • Ideally, same dataset(s) for all…. Absolute values in drug-free condition Time-matched QTc. F Bland Altman plots (slope, Lo. A) Proportion of categorical outliers Create quantitative standards for output QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

Conclusions • • Many of these topics can be studied, thereby allowing unbiased recommendations; The generation of a publicly accessible database comprised of several moxifloxacin / placebo datasets from TQT studies will greatly facilitate this research; Much of this research is ‘precompetitiv’ research – all findings rapidly become public and will not provide anyone individual player competitive edge; Sponsors with experience from many TQT studies can obviously also contribute individually and are encouraged to publish their results. QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D

THANK YOU Borje Darpo MD Ph. D Associate Professor of Cardiology Pharmaceutical Consultant borje. darpo@telia. com QT and arrhythmia issues in drug development. DIA Washington DC, April 2008 Borje Darpo MD Ph. D