Unnatural amino acid mutagenesis for sitespecific incorporation of
- Slides: 1

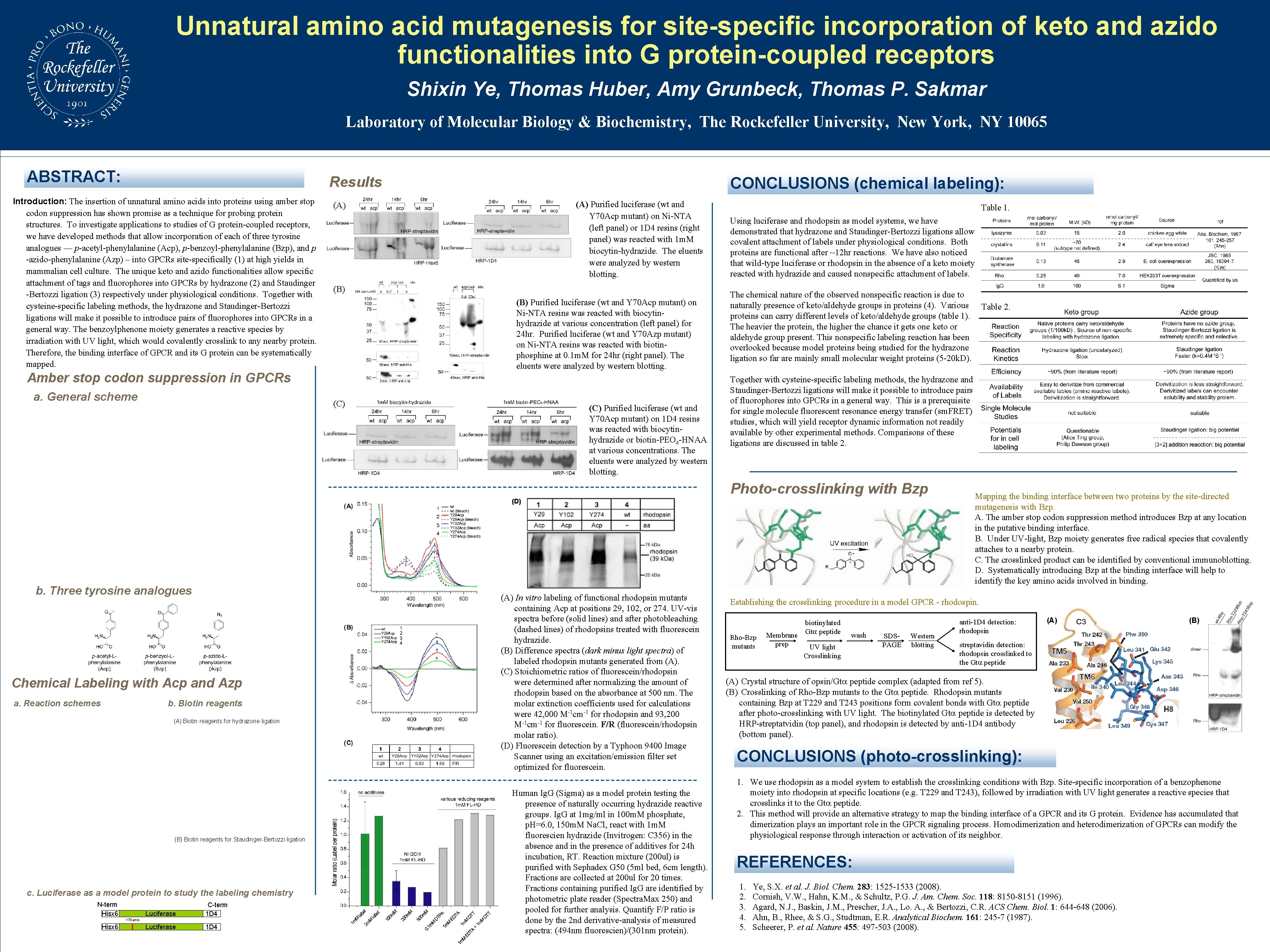
Unnatural amino acid mutagenesis for site-specific incorporation of keto and azido functionalities into G protein-coupled receptors Shixin Ye, Thomas Huber, Amy Grunbeck, Thomas P. Sakmar Laboratory of Molecular Biology & Biochemistry, The Rockefeller University, New York, NY 10065 ABSTRACT: Results Introduction: The insertion of unnatural amino acids into proteins using amber stop codon suppression has shown promise as a technique for probing protein structures. To investigate applications to studies of G protein-coupled receptors, we have developed methods that allow incorporation of each of three tyrosine analogues –– p-acetyl-phenylalanine (Acp), p-benzoyl-phenylalanine (Bzp), and p -azido-phenylalanine (Azp) – into GPCRs site-specifically (1) at high yields in mammalian cell culture. The unique keto and azido functionalities allow specific attachment of tags and fluorophores into GPCRs by hydrazone (2) and Staudinger -Bertozzi ligation (3) respectively under physiological conditions. Together with cysteine-specific labeling methods, the hydrazone and Staudinger-Bertozzi ligations will make it possible to introduce pairs of fluorophores into GPCRs in a general way. The benzoylphenone moiety generates a reactive species by irradiation with UV light, which would covalently crosslink to any nearby protein. Therefore, the binding interface of GPCR and its G protein can be systematically mapped. (A) Purified luciferase (wt and Y 70 Acp mutant) on Ni-NTA (left panel) or 1 D 4 resins (right panel) was reacted with 1 m. M biocytin-hydrazide. The eluents were analyzed by western blotting. (A) (B) Purified luciferase (wt and Y 70 Acp mutant) on Ni-NTA resins was reacted with biocytinhydrazide at various concentration (left panel) for 24 hr. Purified luciferae (wt and Y 70 Azp mutant) on Ni-NTA resins was reacted with biotinphosphine at 0. 1 m. M for 24 hr (right panel). The eluents were analyzed by western blotting. Amber stop codon suppression in GPCRs a. General scheme CONCLUSIONS (chemical labeling): (C) Purified luciferase (wt and Y 70 Acp mutant) on 1 D 4 resins was reacted with biocytinhydrazide or biotin-PEO 4 -HNAA at various concentrations. The eluents were analyzed by western blotting. Table 1. Using luciferase and rhodopsin as model systems, we have demonstrated that hydrazone and Staudinger-Bertozzi ligations allow covalent attachment of labels under physiological conditions. Both proteins are functional after ~12 hr reactions. We have also noticed that wild-type luciferase or rhodopsin in the absence of a keto moiety reacted with hydrazide and caused nonspecific attachment of labels. The chemical nature of the observed nonspecific reaction is due to naturally presence of keto/aldehyde groups in proteins (4). Various Table 2. proteins can carry different levels of keto/aldehyde groups (table 1). The heavier the protein, the higher the chance it gets one keto or aldehyde group present. This nonspecific labeling reaction has been overlooked because model proteins being studied for the hydrazone ligation so far are mainly small molecular weight proteins (5 -20 k. D). Together with cysteine-specific labeling methods, the hydrazone and Staudinger-Bertozzi ligations will make it possible to introduce pairs of fluorophores into GPCRs in a general way. This is a prerequisite for single molecule fluorescent resonance energy transfer (sm. FRET) studies, which will yield receptor dynamic information not readily available by other experimental methods. Comparisons of these ligations are discussed in table 2. Photo-crosslinking with Bzp (D) b. Three tyrosine analogues Chemical Labeling with Acp and Azp a. Reaction schemes b. Biotin reagents (A) Biotin reagents for hydrazone ligation (B) Biotin reagents for Staudinger-Bertozzi ligation c. Luciferase as a model protein to study the labeling chemistry N-term Hisx 6 Luciferase C-term 1 D 4 Luciferase 1 D 4 Y 70 amb Hisx 6 (A) In vitro labeling of functional rhodopsin mutants containing Acp at positions 29, 102, or 274. UV-vis spectra before (solid lines) and after photobleaching (dashed lines) of rhodopsins treated with fluorescein hydrazide. (B) Difference spectra (dark minus light spectra) of labeled rhodopsin mutants generated from (A). (C) Stoichiometric ratios of fluorescein/rhodopsin were determined after normalizing the amount of rhodopsin based on the absorbance at 500 nm. The molar extinction coefficients used for calculations were 42, 000 M-1 cm-1 for rhodopsin and 93, 200 M-1 cm-1 for fluorescein. F/R (fluorescein/rhodopsin molar ratio). (D) Fluorescein detection by a Typhoon 9400 Image Scanner using an excitation/emission filter set optimized for fluorescein. Human Ig. G (Sigma) as a model protein testing the presence of naturally occurring hydrazide reactive groups. Ig. G at 1 mg/ml in 100 m. M phosphate, p. H=6. 0, 150 m. M Na. Cl, react with 1 m. M fluorescien hydrazide (Invitrogen: C 356) in the absence and in the presence of additives for 24 h incubation, RT. Reaction mixture (200 ul) is purified with Sephadex G 50 (5 ml bed, 6 cm length). Fractions are collected at 200 ul for 20 times. Fractions containing purified Ig. G are identified by photometric plate reader (Spectra. Max 250) and pooled for further analysis. Quantify F/P ratio is done by the 2 nd derivative-analysis of measured spectra: (494 nm fluorescien)/(301 nm protein). Mapping the binding interface between two proteins by the site-directed mutagenesis with Bzp. A. The amber stop codon suppression method introduces Bzp at any location in the putative binding interface. B. Under UV-light, Bzp moiety generates free radical species that covalently attaches to a nearby protein. C. The crosslinked product can be identified by conventional immunoblotting. D. Systematically introducing Bzp at the binding interface will help to identify the key amino acids involved in binding. Establishing the crosslinking procedure in a model GPCR - rhodospin. Rho-Bzp mutants Membrane prep biotinylated Gt peptide wash UV light Crosslinking SDSPAGE Western blotting anti-1 D 4 detection: rhodopsin (A) (B) streptavidin detection: rhodopsin crosslinked to the Gt peptide (A) Crystal structure of opsin/Gt peptide complex (adapted from ref 5). (B) Crosslinking of Rho-Bzp mutants to the Gt peptide. Rhodopsin mutants containing Bzp at T 229 and T 243 positions form covalent bonds with Gt peptide after photo-crosslinking with UV light. The biotinylated Gt peptide is detected by HRP-streptatvidin (top panel), and rhodopsin is detected by anti-1 D 4 antibody (bottom panel). CONCLUSIONS (photo-crosslinking): 1. We use rhodopsin as a model system to establish the crosslinking conditions with Bzp. Site-specific incorporation of a benzophenone moiety into rhodopsin at specific locations (e. g. T 229 and T 243), followed by irradiation with UV light generates a reactive species that crosslinks it to the Gt peptide. 2. This method will provide an alternative strategy to map the binding interface of a GPCR and its G protein. Evidence has accumulated that dimerization plays an important role in the GPCR signaling process. Homodimerization and heterodimerization of GPCRs can modify the physiological response through interaction or activation of its neighbor. REFERENCES: 1. 2. 3. 4. 5. Ye, S. X. et al. J. Biol. Chem. 283: 1525 -1533 (2008). Cornish, V. W. , Hahn, K. M. , & Schultz, P. G. J. Am. Chem. Soc. 118: 8150 -8151 (1996). Agard, N. J. , Baskin, J. M. , Prescher, J. A. , Lo. A. , & Bertozzi, C. R. ACS Chem. Biol. 1: 644 -648 (2006). Ahn, B. , Rhee, & S. G. , Studtman, E. R. Analytical Biochem. 161: 245 -7 (1987). Scheerer, P. et al. Nature 455: 497 -503 (2008).

