Unmet Needs of Korea T 2 DM and






























- Slides: 68

Unmet Needs of Korea T 2 DM and Role of Forxiga & Xigduo ATLAS ID 456. 308, 022 l Exp. 20190310

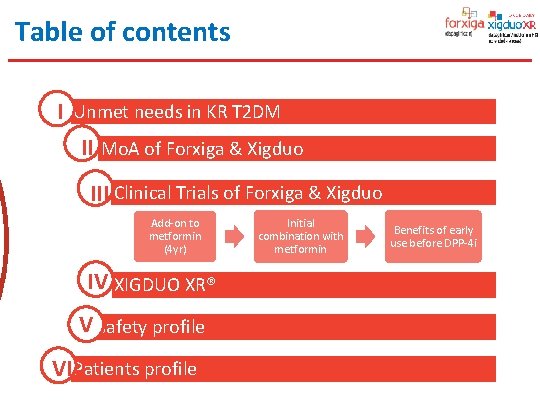
Table of contents I Unmet needs in KR T 2 DM II Mo. A of Forxiga & Xigduo III Clinical Trials of Forxiga & Xigduo Add-on to metformin (4 yr) IV XIGDUO XR® V Safety profile VI Patients profile Initial combination with metformin Benefits of early use before DPP-4 i

Unmet needs in KR T 2 DM

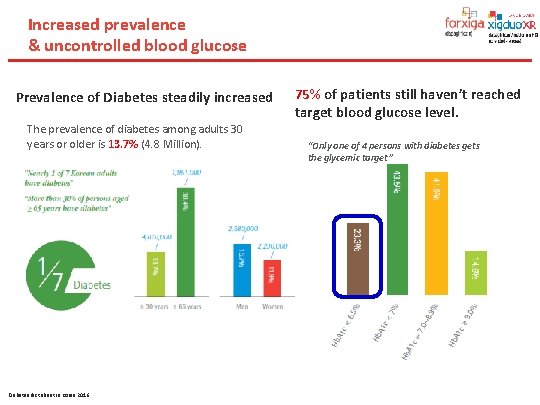
Increased prevalence & uncontrolled blood glucose Prevalence of Diabetes steadily increased The prevalence of diabetes among adults 30 years or older is 13. 7% (4. 8 Million). Diabetes fact sheet in Korea 2016 75% of patients still haven’t reached target blood glucose level. “Only one of 4 persons with diabetes gets the glycemic target”

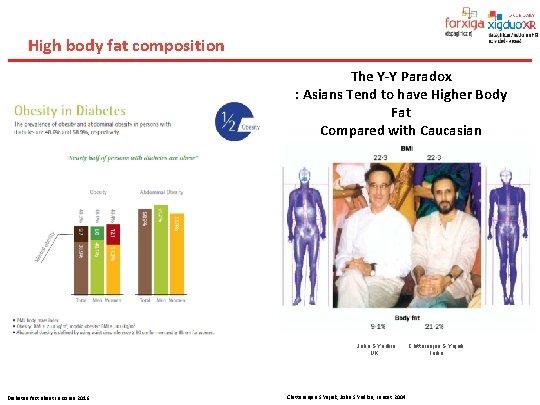
High body fat composition The Y-Y Paradox : Asians Tend to have Higher Body Fat Compared with Caucasian John S Yudkin UK Diabetes fact sheet in Korea 2016 Chittaranjan S Yajnik, John S Yudkin, Lancet 2004 Chittaranjan S Yajnik India

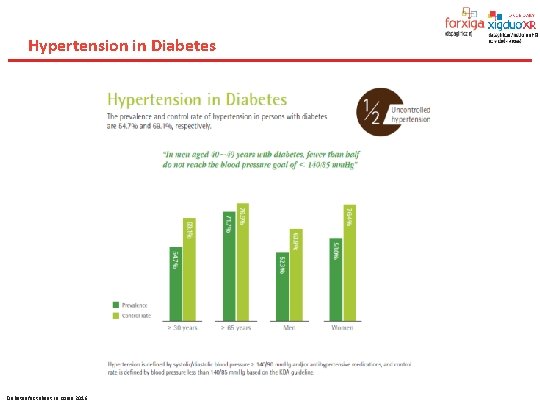
Hypertension in Diabetes fact sheet in Korea 2016

Korean T 2 DM patients need beta-cell preservation treatment Selective -Cell Loss and -Cell Expansion in T 2 DM in Korea Selective - cell loss -cell expansion The pattern of distribution of -cell (%) Decreased -cell but increased -cell proportion in the islets suggests for a selective -cell loss in the pathogenesis of Korean type 2 diabetes. Yoon GH et al. , JCEM, 2003

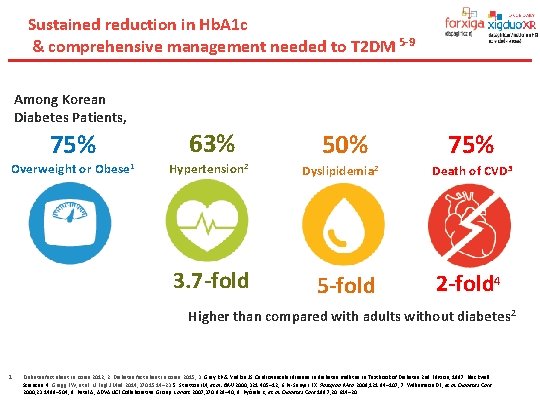
Sustained reduction in Hb. A 1 c & comprehensive management needed to T 2 DM 5 -9 Among Korean Diabetes Patients, 75% Overweight or Obese 1 63% 50% Hypertension 2 Dyslipidemia 2 3. 7 -fold 5 -fold 75% Death of CVD 3 2 -fold 4 Higher than compared with adults without diabetes 2 1. Diabetes fact sheet in Korea 2012, 2. Diabetes fact sheet in Korea 2015, 3. Gray RP & Yudkin JS. Cardiovascular disease in diabetes mellitus. In Textbook of Diabetes 2 nd Edition, 1997. Blackwell Sciences. 4. Gregg EW, et al. N Engl J Med 2014; 370: 1514– 23 5. Stratton IM, et al. BMJ 2000; 321: 405– 12; 6. Pi-Sunyer FX. Postgrad Med 2009; 121: 94– 107; 7. Williamson DF, et al. Diabetes Care 2000; 23: 1499– 504; 8. Patel A, ADVANCE Collaborative Group. Lancet 2007; 370: 829– 40; 9. Pyǒrälä K, et al. Diabetes Care 1997; 20: 614– 20.

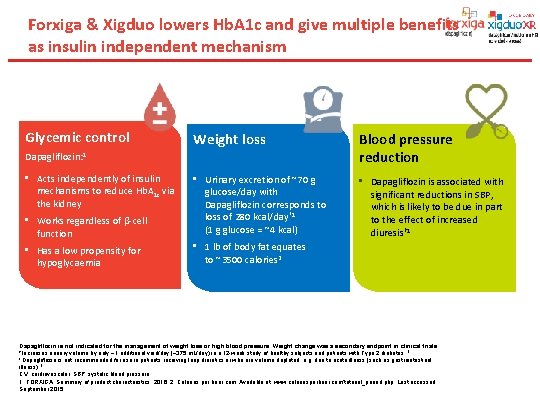
Forxiga & Xigduo lowers Hb. A 1 c and give multiple benefits as insulin independent mechanism Glycemic control Weight loss Blood pressure reduction • Works regardless of β-cell function • Urinary excretion of ~70 g glucose/day with Dapagliflozin corresponds to loss of 280 kcal/day*1 (1 g glucose = ~4 kcal) • Dapagliflozin is associated with significant reductions in SBP, which is likely to be due in part to the effect of increased diuresis† 1 • Has a low propensity for hypoglycaemia • 1 lb of body fat equates to ~3500 calories 2 Dapagliflozin: 1 • Acts independently of insulin mechanisms to reduce Hb. A 1 c via the kidney Dapagliflozin is not indicated for the management of weight loss or high blood pressure. Weight change was a secondary endpoint in clinical trials. *Increases urinary volume by only ~1 additional void/day (~375 m. L/day) in a 12 -week study of healthy subjects and patients with Type 2 diabetes. 1 †Dapagliflozin is not recommended for use in patients receiving loop diuretics or who are volume depleted, e. g. due to acute illness (such as gastrointestinal illness). 1 CV, cardiovascular; SBP, systolic blood pressure. 1. FORXIGA. Summary of product characteristics, 2016; 2. Calories per hour. com. Available at: www. caloriesperhour. com/tutorial_pound. php. Last accessed September 2015.

Mo. A of Forxiga & Xigduo

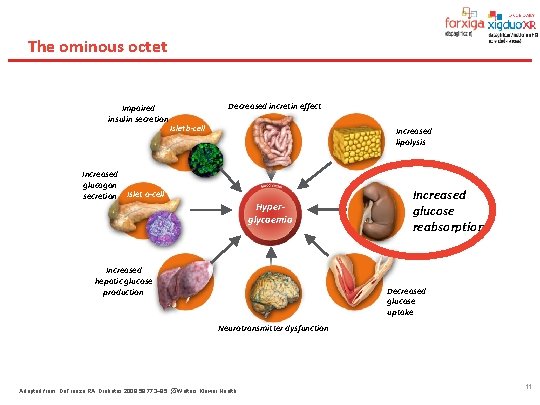
The ominous octet Impaired insulin secretion Decreased incretin effect Islet b-cell Increased lipolysis Increased glucagon secretion Islet a-cell Hyperglycaemia Increased hepatic glucose production Increased glucose reabsorption Decreased glucose uptake Neurotransmitter dysfunction Adapted from: De. Fronzo RA. Diabetes 2009; 58: 773– 95. Wolters Kluwer Health 11

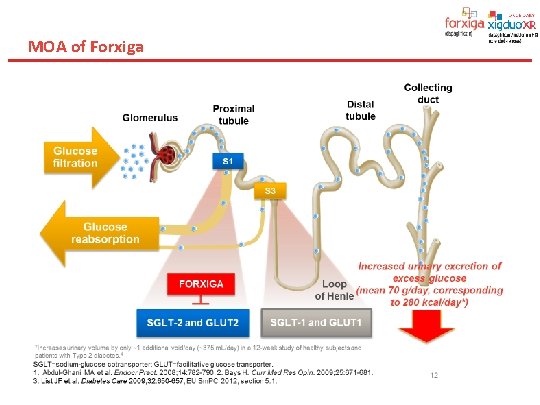
MOA of Forxiga

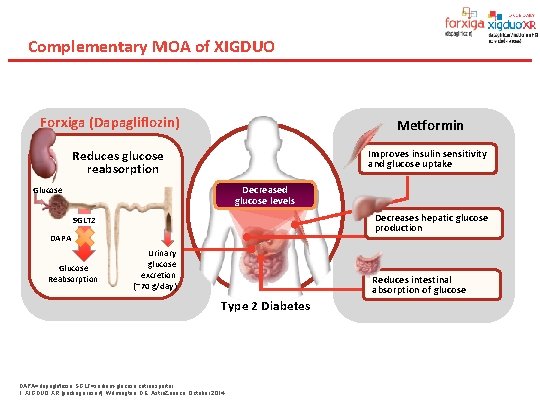
Complementary MOA of XIGDUO Forxiga (Dapagliflozin) Metformin Improves insulin sensitivity and glucose uptake Reduces glucose reabsorption Decreased glucose levels Glucose Decreases hepatic glucose production SGLT 2 DAPA Glucose Reabsorption Urinary glucose excretion (~70 g/day) Reduces intestinal absorption of glucose Type 2 Diabetes DAPA=dapagliflozin; SGLT=sodium-glucose cotransporter. 1. XIGDUO XR [package insert]. Wilmington, DE: Astra. Zeneca; October 2014.

Initial combination with metformin

Initial Combination Therapy: Study design and patient characteristics Objective: Evaluate the efficacy and safety of DAPA + MET XR in treatment-naive adult patients whose type 2 diabetes cannot be controlled with diet and exercise alone 2 Patient Population 2 n Adults (18– 77 years) with 10 -mg Study N=638 TREATMENT NAIVE A 1 C 7. 5%– 12. 0% RANDOMIZATION 24 Weeks DAPA 10 mg + MET XR (n=211) DAPA 10 mg + placebo (n=219) MET XR + placebo (n=208) type 2 diabetes n BMI ≤ 45 kg/m 2 n Key exclusion criteria: serum creatinine ≥ 1. 5 mg/d. L for men or ≥ 1. 4 mg/d. L for women, consistent with metformin labeling Primary End Point 2 n Change in A 1 C from baseline Select Secondary End Points n Change in total body weight from baseline n Noninferiority of DAPA to MET XR for A 1 C – If noninferiority shown, superiority tested for 10 -mg dose • XIGDUO XR was approved based on clinical trials that evaluated dapagliflozin and metformin XR as separate tablets. Bioequivalence of XIGDUO XR to dapagliflozin and metformin XR coadministered as individual tablets was demonstrated in healthy subjects. XR=extended release; DAPA=dapagliflozin; MET=metformin; BMI=body mass index. 1. XIGDUO XR Prescribing Information 2. Henry RR et al. Int J Clin Pract. 2012; 66: 446 -456.

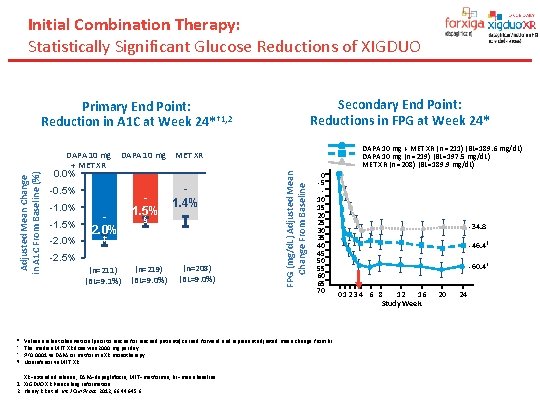
Initial Combination Therapy: Statistically Significant Glucose Reductions of XIGDUO Secondary End Point: Reductions in FPG at Week 24* Primary End Point: Reduction in A 1 C at Week 24*† 1, 2 DAPA 10 mg + MET XR (n =211) (BL=189. 6 mg/d. L) DAPA 10 mg (n =219) (BL=197. 5 mg/d. L) MET XR (n =208) (BL=189. 9 mg/d. L) 0. 0% -0. 5% -1. 0% -1. 5% -2. 0% 1. 5% 1. 4% § ‡ -2. 5% (n=211) (BL=9. 1%) (n=219) (BL=9. 0%) (n=208) (BL=9. 0%) FPG (mg/d. L) Adjusted Mean Change From Baseline Adjusted Mean Change in A 1 C From Baseline (%) DAPA 10 mg MET XR + MET XR 0 -5 10152025303540455055606570 * Values are last observation (prior to rescue for rescued patients) carried forward and represent adjusted mean change from BL. † The median MET XR dose was 2000 mg per day. ‡ P<0. 0001 vs DAPA or metformin XR monotherapy. § Noninferior vs MET XR. XR=extended release; DAPA=dapagliflozin; MET=metformin; BL=mean baseline. 1. XIGDUO XR Prescribing Information 2. Henry RR et al. Int J Clin Pract. 2012; 66: 446 -456. -34. 8 -46. 4‡ -60. 4† 0 123 4 6 8 12 16 Study Week 20 24

Initial Combination Therapy: Change in Weight and Blood Pressure of XIGDUO DAPA 10 mg + MET XR -4. 0 -1. 4 kg -3. 3 kg† DAPA 10 mg + MET XR 0. 0 -2. 0 Reductions in Systolic BP at Week 24* -2. 7 kg† Adjusted Mean Change From Baseline (mm. Hg) Adjusted Mean Change in Body Weight From Baseline (lb) Secondary End Point: Reductions in Body Weight at Week 24* DAPA 10 mg 0. 0 -1. 2 mm. Hg -1. 0 -2. 0 -3. 0 MET XR -3. 3 mm. Hg -4. 0 -5. 0 -6. 0 (n=182) (BL=127. 6 mm. Hg) -8. 0 (n=209) (BL=88. 6 kg) (n=219) (BL=88. 5 kg) (n=208) (BL=87. 2 kg) XIGDUO XR is not indicated for weight loss. (n=185) (BL=127. 8 mm. Hg) (n=179) (BL=130. 6 mm. Hg) XIGDUO XR is not indicated for the treatment of hypertension * Values are last observation (prior to rescue for rescued patients) carried forward and represent adjusted mean change from BL. † P<0. 0001 vs MET XR. XR=extended release; DAPA=dapagliflozin; MET=mean metformin; BL=mean baseline. 1. XIGDUO XR Prescribing Information 2. Henry RR et al. Int J Clin Pract. 2012; 66: 446 -456.

Add-on to metformin

XIGDUO vs. placebo: Study design and patient characteristics Baseline characteristics 1 • • Phase III, multicentre, double-blind, parallel-group, placebo-controlled trial to evaluate the efficacy and safety profile of FORXIGA in 546 patients with Type 2 diabetes uncontrolled on metformin 1 Primary endpoint: Change from baseline in Hb. A 1 c at 24 weeks 1 ( Open-label pre-study metformin dosing FORXIGA 10 mg (n=135) 53. 7 52. 7 Male sex (%) 76 77 BMI (kg/m 2) 31. 8 31. 2 Mean duration of Type 2 diabetes (years) 5. 8 6. 1 Hb. A 1 c (%) 8. 11 7. 92 Serum creatinine (µmol/L) 77. 3 77. 5 Urinary glucose/creatinine ratio (g/g) 3. 4 0. 9 FORXIGA 2. 5 mg* (n=137), 5 mg (n=137) or 10 mg* (n=135) once daily Placebo (n=137) Extension 2 Double-blind 1 0 weeks Mean age (years) Placebo (n=137) 24 weeks *Note that the 2. 5 mg dose is unlicensed and only data for the 10 mg dose group are shown in this deck. BMI, body mass index. 1. Bailey CJ, et al. Lancet 2010; 375: 2223– 33; 2. Bailey CJ, et al. BMC Med 2013; 11: 43. 102 weeks

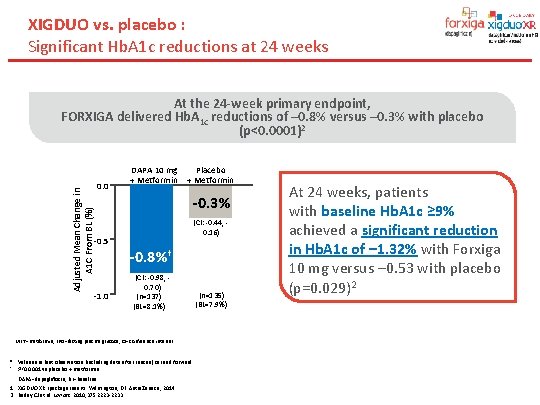
XIGDUO vs. placebo : Significant Hb. A 1 c reductions at 24 weeks At the 24 -week primary endpoint, versus – 0. 3% with placebo Adjusted Mean Change in A 1 C From BL (%) Primary End Point: FORXIGA delivered Hb. A 1 c reductions of – 0. 8% Reductions in A 1 C at Week 24* (p<0. 0001)2 0. 0 -0. 5 -1. 0 DAPA 10 mg + Metformin Placebo + Metformin -0. 3% -0. 7%† (CI: -0. 44, 0. 16) -0. 8%† (CI: -0. 98, 0. 70) (n=137) (BL=8. 1%) MET=metformin; FPG=fasting plasma glucose; CI=confidence interval. * Values are last observation (including data after rescue) carried forward. † P<0. 0001 vs placebo + metformin. DAPA=dapagliflozin; BL=baseline. 1. XIGDUO XR [package insert]. Wilmington, DE: Astra. Zeneca; 2014. 2. Bailey CJ et al. Lancet. 2010; 375: 2223 -2233. (n=135) (BL=7. 9%) At 24 weeks, patients with baseline Hb. A 1 c ≥ 9% achieved a significant reduction in Hb. A 1 c of – 1. 32% with Forxiga 10 mg versus – 0. 53 with placebo (p=0. 029)2

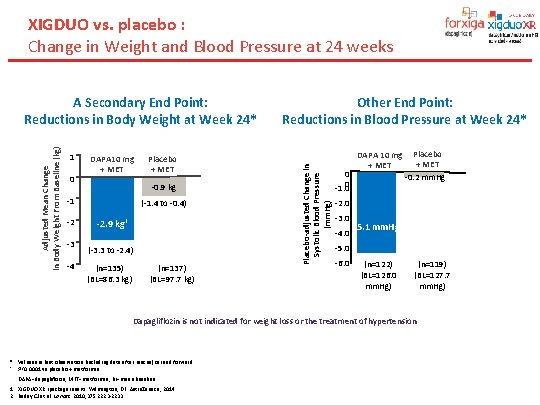
XIGDUO vs. placebo : Change in Weight and Blood Pressure at 24 weeks 1 0 DAPA 10 mg + MET -0. 9 kg -1 -2 -3 -4 Placebo + MET (-1. 4 to -0. 4) -2. 9 kg† (-3. 3 to -2. 4) (n=135) (BL=86. 3 kg) (n=137) (BL=97. 7 kg) Other End Point: Reductions in Blood Pressure at Week 24* Placebo-adjusted Change in Systolic Blood Pressure (mm. Hg) Adjusted Mean Change in Body Weight From Baseline (kg) A Secondary End Point: Reductions in Body Weight at Week 24* 0. 0 -1. 0 DAPA 10 mg + MET Placebo + MET -0. 2 mm. Hg -2. 0 -3. 0 -4. 0 -5. 1 mm. Hg -5. 0 -6. 0 (n=122) (BL=126. 0 mm. Hg) (n=119) (BL=127. 7 mm. Hg) Dapagliflozin is not indicated for weight loss or the treatment of hypertension * Values are last observation (including data after rescue) carried forward. † P<0. 0001 vs placebo + metformin. DAPA=dapagliflozin; MET=metformin; BL=mean baseline. 1. XIGDUO XR [package insert]. Wilmington, DE: Astra. Zeneca; 2014. 2. Bailey CJ et al. Lancet. 2010; 375: 2223 -2233.

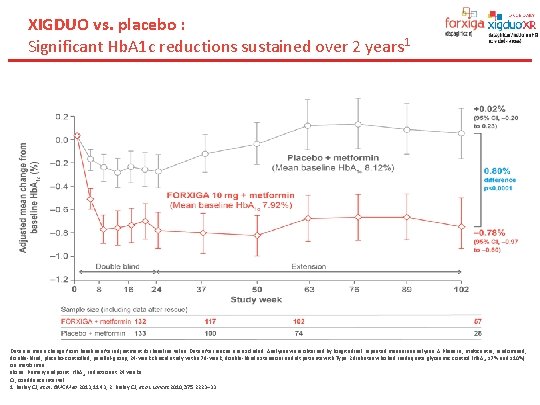
XIGDUO vs. placebo : Significant Hb. A 1 c reductions sustained over 2 years 1 Data are mean change from baseline after adjustment for baseline value. Data after rescue are excluded. Analyses were obtained by longitudinal repeated measures analyses. A Phase III, multicentre, randomised, double-blind, placebo-controlled, parallel-group, 24 -week clinical study with a 78 -week, double-blind extension in adult patients with Type 2 diabetes who had inadequate glycaemic control (Hb. A 1 c ≥ 7% and ≤ 10%) on metformin alone. Primary endpoint: Hb. A 1 c reduction at 24 weeks. CI, confidence interval. 1. Bailey CJ, et al. BMC Med 2013; 11: 43; 2. Bailey CJ, et al. Lancet 2010; 375: 2223– 33.

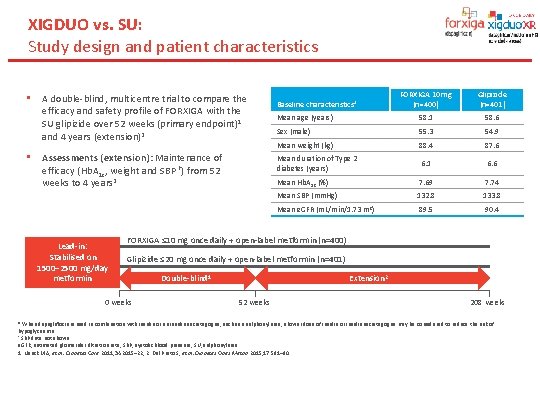
XIGDUO vs. SU: Study design and patient characteristics • • A double-blind, multicentre trial to compare the efficacy and safety profile of FORXIGA with the SU glipizide over 52 weeks (primary endpoint)1 and 4 years (extension)2 Assessments (extension): Maintenance of efficacy (Hb. A 1 c, weight and SBP †) from 52 weeks to 4 years 2 Lead-in: Stabilised on 1500– 2500 mg/day metformin FORXIGA 10 mg (n=400) Glipizide (n=401) Mean age (years) 58. 1 58. 6 Sex (male) 55. 3 54. 9 Mean weight (kg) 88. 4 87. 6 Mean duration of Type 2 diabetes (years) 6. 1 6. 6 Baseline characteristics 2 Mean Hb. A 1 c (%) 7. 69 7. 74 Mean SBP (mm. Hg) 132. 8 133. 8 Mean e. GFR (m. L/min/1. 73 m 2) 89. 5 90. 4 FORXIGA ≤ 10 mg once daily + open-label metformin (n=400) Glipizide ≤ 20 mg once daily + open-label metformin (n=401) 0 weeks Extension 2 Double-blind 1 52 weeks 208 weeks * When dapagliflozin is used in combination with insulin or an insulin secretagogue, such as a sulphonylurea, a lower dose of insulin or insulin secretagogue may be considered to reduce the risk of hypoglycaemia † SBP data not shown. e. GFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SU, sulphonylurea. 1. Nauck MA, et al. Diabetes Care 2011; 34: 2015– 22; 2. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581– 90.

XIGDUO vs. SU: Comparable Hb. A 1 c reductions over 4 years 1 At 52 weeks, reductions in Hb. A 1 c were statistically non-inferior to glipizide (– 0. 52% for both) 2 (Del Prato 2015)(/p 6/para 1) Data are adjusted mean change from baseline derived from a longitudinal repeated measures mixed model. A Phase III, multicentre, randomised, double-blind, parallel-group, 52 -week, glipizide-controlled, non-inferiority study with a double-blind extension to evaluate the efficacy and safety profile of FORXIGA 10 mg + metformin (1500– 2500 mg/day) versus glipizide + metformin (1500– 2500 mg/day) in patients with inadequate glycaemic control (Hb. A 1 c >6. 5% and ≤ 10%) on metformin alone. 1 CI, confidence interval. 1. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581– 90; 2. Nauck MA, et al. Diabetes Care 2011; 34: 2015– 22.

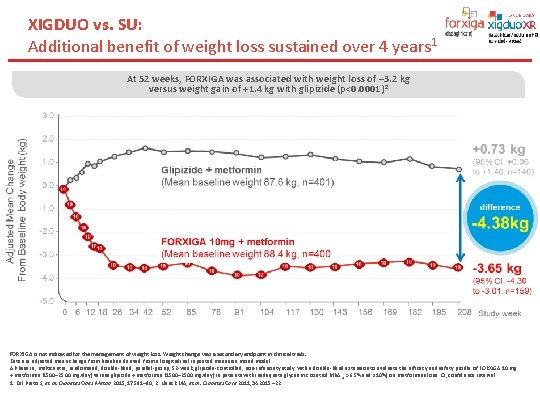
XIGDUO vs. SU: Additional benefit of weight loss sustained over 4 years 1 At 52 weeks, FORXIGA was associated with weight loss of – 3. 2 kg versus weight gain of +1. 4 kg with glipizide (p<0. 0001) 2 FORXIGA is not indicated for the management of weight loss. Weight change was a secondary endpoint in clinical trials. Data are adjusted mean change from baseline derived from a longitudinal repeated measures mixed model. A Phase III, multicentre, randomised, double-blind, parallel-group, 52 -week, glipizide-controlled, non-inferiority study with a double-blind extension to evaluate the efficacy and safety profile of FORXIGA 10 mg + metformin (1500– 2500 mg/day) versus glipizide + metformin (1500– 2500 mg/day) in patients with inadequate glycaemic control (Hb. A 1 c >6. 5% and ≤ 10%) on metformin alone. CI, confidence interval. 1. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581– 90; 2. Nauck MA, et al. Diabetes Care 2011; 34: 2015– 22

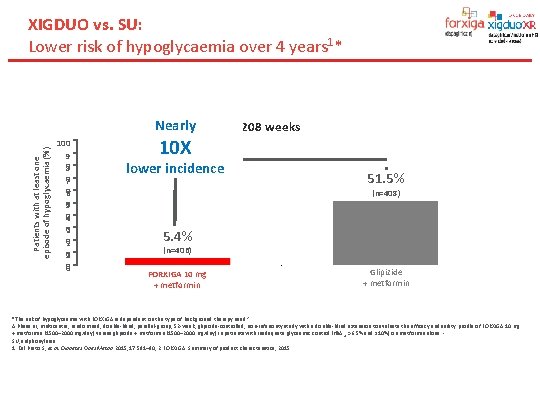
XIGDUO vs. SU: Lower risk of hypoglycaemia over 4 years 1* Patients with at least one episode of hypoglycaemia (%) Nearly 100 9 0 8 0 7 0 6 0 5 0 4 0 3 0 2 0 10 X lower incidence 208 weeks 51. 5% (n=408) 5. 4% (n=406) FORXIGA 10 mg + metformin Glipizide + metformin *The risk of hypoglycaemia with FORXIGA is dependent on the type of background therapy used. 2 A Phase III, multicentre, randomised, double-blind, parallel-group, 52 -week, glipizide-controlled, non-inferiority study with a double-blind extension to evaluate the efficacy and safety profile of FORXIGA 10 mg + metformin (1500– 2000 mg/day) versus glipizide + metformin (1500– 2000 mg/day) in patients with inadequate glycaemic control (Hb. A 1 c >6. 5% and ≤ 10%) on metformin alone. 1 SU, sulphonylurea. 1. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581– 90; 2. FORXIGA. Summary of product characteristics, 2015.

be More, #B 4 DPP 4

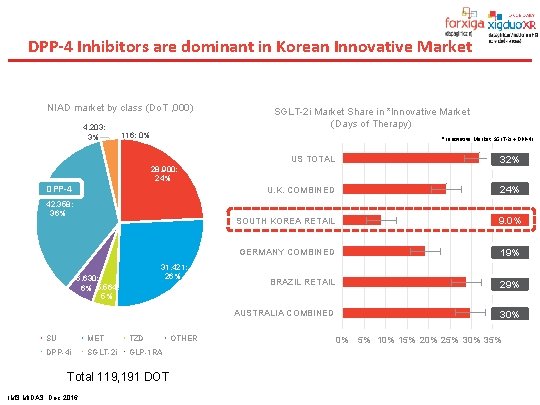
DPP-4 Inhibitors are dominant in Korean Innovative Market NIAD market by class (Do. T , 000) 4, 203; 3% SGLT-2 i Market Share in *Innovative Market (Days of Therapy) 116; 0% * Innovative Market: SGLT-2 i + DPP-4 i US TOTAL 32% U. K. COMBINED 24% SOUTH KOREA RETAIL 9. 0% GERMANY COMBINED 19% BRAZIL RETAIL 29% AUSTRALIA COMBINED 30% 28, 900; 24% DPP-4 42, 358; 36% 31, 421; 26% 6, 630; 6% 5, 564; 5% SU MET TZD DPP-4 i SGLT-2 i GLP-1 RA Total 119, 191 DOT IMS MIDAS, Dec 2016 OTHER 0% 5% 10% 15% 20% 25% 30% 35%

Superior Hb. A 1 c reductions with Forxiga compared with a DPP 4 inhibitor Adjusted Mean Change in A 1 C From BL (%) At the 24 -week primary endpoint, FORXIGA delivered Hb. A 1 c reductions of – 1. 2% versus – 0. 9% with saxagliptin (p<0. 004) Dapagliflozin 10 mg added on to metformin XR Saxagliptin 5 mg added on to metformin XR (n=151*) (n=143*) 0. 0 -0. 5 -0. 7%† -1. 20% -1. 0 -0. 88% (CI: -1. 03, -0. 72) 0. 32% (CI: -1. 35, -1. 04) difference (CI: -0. 10, -0. 54) CI=confidence interval. -1. 5 • • • Baseline Hb. A 1 C=8. 87% Baseline Hb. A 1 C=9. 03% This was a phase III, 24 -week, multi-center, randomized, double-blind, active controlled parallel-group study to compare the efficacy and safety of the dual add-on of saxagliptin 5 mg and dapagliflozin 10 mg with either saxagliptin 5 mg or dapagliflozin 10 mg added on alone in adult patients with Type 2 diabetes who had inadequate glycaemic control (Hb. A 1 c ≥ 8% to ≤ 12%) on metformin alone. The study met its primary endpoint. This is a retrospective post hoc analysis of the data. Mean reduction in weight with FORXIGA + metformin XR was – 2. 4 kg vs. no change from baseline in patients treated with saxagliptin + metformin XR. Mean reduction in systolic blood pressure with FORXIGA + metformin XR was – 3. 5 mm. Hg vs. an increase of 0. 3 mm. Hg from baseline in patients treated with saxagliptin + metformin XR. * Number of randomized patients with non-missing baseline values and Week 24 last observation carried forward (LOCF) 1. Rosenstock, et al. Diabetes Care 2015; 38: 376– 383 2. Rosenstock J, et al. WCIRDC 2016 Poster 99

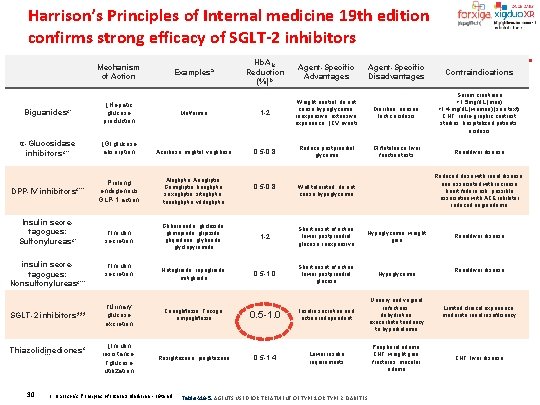
Harrison’s Principles of Internal medicine 19 th edition confirms strong efficacy of SGLT-2 inhibitors Mechanism of Action Hb. A 1 c Reduction (%) b Agent-Specific Advantages Agent-Specific Disadvantages Contraindications Metformin 1 -2 Weight neutral, do not cause hypoglycemia, inexpensive, extensive experience, ↓CV events Diarrhea, nausea, lactic acidosis Serum creatinine >1. 5 mg/d. L (men) >1. 4 mg/d. L (women) (see text), CHF, radio-graphic contrast studies, hospitalized patients, acidosis 0. 5 -0. 8 Reduce postprandial glycemia Gl flatulence, liver function tests Renal/liver disease Examples a Biguanidesc* ↓Hepatic glucose production α-Glucosidase inhibitorsc** ↓Gl glucose absorption Acarbose, miglitol, voglibose Prolong endogenous GLP-1 action Alogliptin, Anagliptin, Gemigliptin, linagliptin, saxagliptin, sitagliptin, teneligliptin, vildagliptin ↑Insulin secretion Glibornuride, gliclazide, glimepiride, glipizide, gliquidone, glyburide, glyclopyramide 1 -2 Short onset of action, lower postprandial glucose, inexpensive Hypoglycemia, weight gain Nateglinide, repaglinide, mitiglinide 0. 5 -1. 0 Short onset of action, lower postprandial glucose Hypoglycemia Urinary and vaginal infections, dehydration, exacerbate tendency to hyperkalemia Peripheral edema, CHF, weight gain, fractures, macular edema DPP-IV inhibitors c*** Insulin secretagogues: Sulfonylureas c* insulin secretagogues: Nonsulfonylureas c*** SGLT-2 inhibitors*** Thiazolidinediones c *** 30 ↑Insulin secretion 0. 5 -0. 8 ↑Urinary glucose excretion Canagliflozin, Forxiga, empagliflozin 0. 5 -1. 0 Insulin secretion and action independent ↓Insulin resistance, ↑glucose utilization Rosiglitazone, pioglitazone 0. 5 -1. 4 Lower insulin requirements 1. Harrison's Principles of Internal Medicine - 19 th ed Reduced dose with renal disease; one associated with increase heart failure risk; possible association with ACE inhibitor induced angioedema Well tolerated, do not cause hypoglycemia Table 418 -5. AGENTS USED FOR TREATMENT OF TYPE 1 OR TYPE 2 DIABETES Renal/liver disease Limited clinical experience; moderate renal insufficiency CHF, liver disease

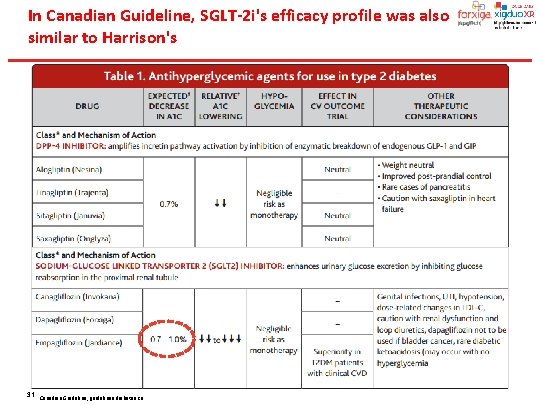
In Canadian Guideline, SGLT-2 i's efficacy profile was also similar to Harrison's 31 Canadian Guideline, guidelines. diabetes. ca

AACE guideline recommend SGLT 2 i as a 1 st choice after MET as Oral NIAD AACE, American Association of Clinical Endocrinologists; ACE, American College of Endocrinology; AGi, alpha-glucosidase inhibitor; DPP 4 i, dipeptidyl peptidase-4 inhibitor; GLN, glinides; GLP-1 RA, glucagon-like peptide-1 receptor agonist; QR, quick release; SGLT 2 i, sodium-glucose co-transporter-2 inhibitor; SU, sulphonylurea; TZD, thiazolidinedione; NIAD: Non-insulin anti diabetic drug 1. Garber AJ, et al. Endocr Pract 2016; 22: 84 -113.

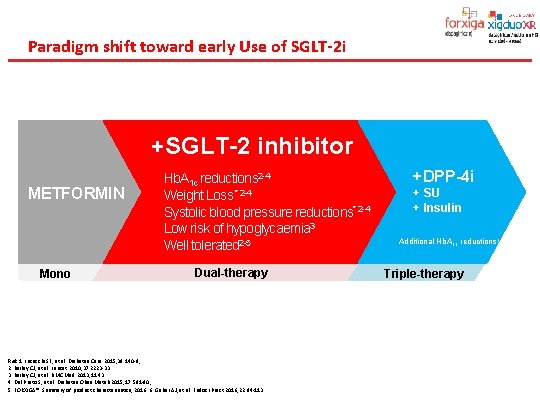
Paradigm shift toward early Use of SGLT-2 i +SGLT-2 inhibitor METFORMIN Mono Hb. A 1 c reductions 2 -4 Weight Loss * 2 -4 Systolic blood pressure reductions* 2 -4 Low risk of hypoglycaemia 3 Well tolerated 2 -5 Dual-therapy Ref. 1. Inzucchi SE, et al. Diabetes Care 2015; 38: 140 -9, 2. Bailey CJ, et al. Lancet 2010; 37: 2223 -33. 3. Bailey CJ, et al. BMC Med 2013; 11: 43. 4. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581 -90, 5. FORXIGA®. Summary of product characteriastics, 2016. 6. Gaber AJ, et al. Endocr Pract 2016; 22: 84 -113. +DPP-4 i + SU + Insulin Additional Hb. A 1 c reductions 1, 6 Triple-therapy

XIGDUO XR®

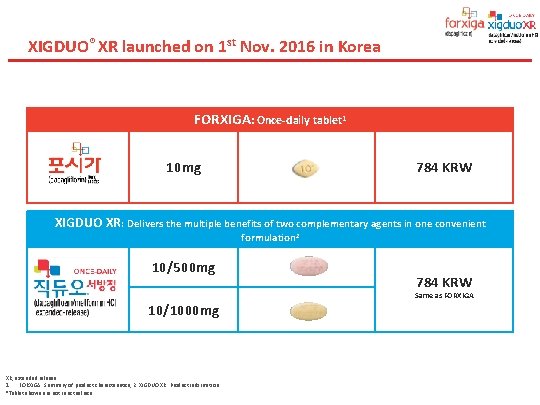
XIGDUO® XR launched on 1 st Nov. 2016 in Korea 35 브랜드명 XIGDUO XR (dapagliflozin/metformin XR) / 직듀오 서방정 제형 10/500 mg and 10/1000 mg 보험약가 784 원으로 포시가와 동일 보험코드 10/500 mg : 650700960 10/1000 mg : 650700930

XIGDUO® XR launched on 1 st Nov. 2016 in Korea FORXIGA: Once-daily tablet 1 10 mg 784 KRW XIGDUO XR: Delivers the multiple benefits of two complementary agents in one convenient formulation 2 10/500 mg 784 KRW Same as FORXIGA 10/1000 mg . XR, extended release. 1. FORXIGA. Summary of product characteristics, 2. XIGDUO XR. Product information. *Tablet shown are not in actual size

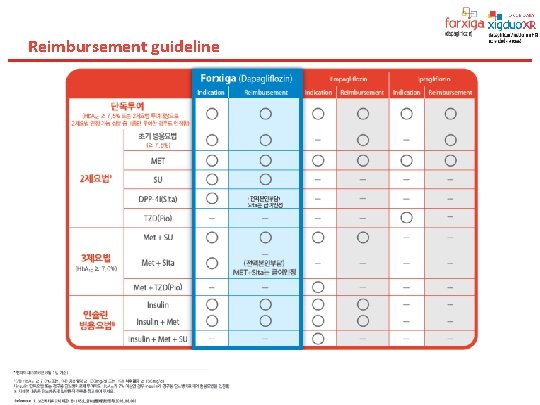
Reimbursement guideline

Reimbursement guideline

Consider delivering multiple benefits earlier with FORXIGA, as first add-on to metformin, or XIGDUO XR Monotherapy Initial combination 1 st Add On DPP-4 Is SU , Insulin…

Safety Profile

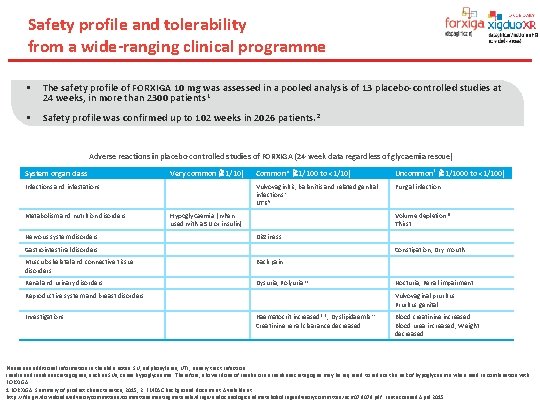
Safety profile and tolerability from a wide-ranging clinical programme § The safety profile of FORXIGA 10 mg was assessed in a pooled analysis of 13 placebo-controlled studies at 24 weeks, in more than 2300 patients 1 § Safety profile was confirmed up to 102 weeks in 2026 patients. 2 Adverse reactions in placebo-controlled studies of FORXIGA (24 -week data regardless of glycaemia rescue) System organ class Very common ( 1/10) Infections and infestations Metabolism and nutrition disorders Nervous system disorders Common* ( 1/100 to <1/10) Uncommon† ( 1/1000 to <1/100) Vulvovaginitis, balanitis and related genital infections‡ UTIs§ Fungal infection Hypoglycaemia (when used with a SU or insulin) Volume depletion¶ Thirst Dizziness Gastrointestinal disorders Constipation, Dry mouth Musculoskeletal and connective tissue disorders Back pain Renal and urinary disorders Dysuria, Polyuria|| Reproductive system and breast disorders Investigations Nocturia, Renal impairment Vulvovaginal pruritus Pruritus genital Haematocrit increased**, Dyslipidaemia†† Creatinine renal clearance decreased Blood creatinine increased Blood urea increased, Weight decreased Please see additional information in the slide notes. SU, sulphonylurea; UTI, urinary tract infection. Insulin and insulin secretagogues, such as SUs, cause hypoglycaemia. Therefore, a lower dose of insulin or an insulin secretagogue may be required to reduce the risk of hypoglycaemia when used in combination with FORXIGA. 1. FORXIGA. Summary of product characteristics, 2015; 2. EMDAC background document. Available at: http: //fda. giv/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm 378079. pdf. Last accessed April 2015.

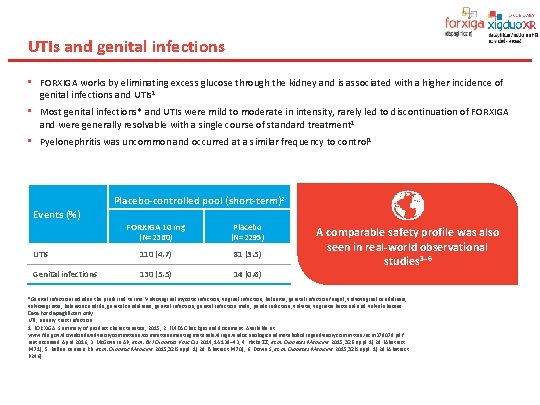
UTIs and genital infections • FORXIGA works by eliminating excess glucose through the kidney and is associated with a higher incidence of genital infections and UTIs 1 • Most genital infections* and UTIs were mild to moderate in intensity, rarely led to discontinuation of FORXIGA and were generally resolvable with a single course of standard treatment 1 • Pyelonephritis was uncommon and occurred at a similar frequency to control 1 Events (%) Placebo-controlled pool (short-term)2 FORXIGA 10 mg (N=2360) Placebo (N=2295) UTIs 110 (4. 7) 81 (3. 5) Genital infections 130 (5. 5) 14 (0. 6) A comparable safety profile was also seen in real-world observational studies 3– 6 *Genital infection includes the preferred terms: Vulvovaginal mycotic infection, vaginal infection, balanitis, genital infection fungal, vulvovaginal candidiasis, vulvovaginitis, balanitis candida, genital candidiasis, genital infection male, penile infection, vulvitis, vaginitis bacterial and vulval abscess. Data for dapagliflozin only UTI, urinary tract infection. 1. FORXIGA. Summary of product characteristics, 2015; 2. EMDAC background document. Available at: www. fda. gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm 378079. pdf. Last accessed April 2016; 3. Mc. Govern AP, et al. Br J Diabetes Vasc Dis 2014; 14: 138– 43; 4. Hitke ZZ, et al. Diabetes Medicine 2015; 32(Suppl. 1): 29 (Abstract P 471); 5. Bellan Kannan RB. et al. Diabetic Medicine 2015; 32(Suppl. 1): 29 (Abstract P 470); 6. Down S, et al. Diabetes Medicine 2015; 32(Suppl. 1): 29 (Abstract P 246).

CV safety profile in a variety of patients • In a subgroup analysis of patients grouped by their degree of CV risk, FORXIGA demonstrated a consistent CV safety profile in patients with: 1 • Established CVD • Differing numbers of prior CVD events • A separate analysis showed that the efficacy of FORXIGA in reducing Hb. A 1 c, SBP and bodyweight over 2 years together with its safety profile demonstrates that FORXIGA is appropriate for use in high-risk patients with Type 2 diabetes and CVD 2 • A randomised outcomes trial to assess the effects of FORXIGA on long-term CV event rate is ongoing 3 DECLARE No. of patients enrolled 17, 150 (planned) Duration of study 6 years Primary endpoint Time to first event included in the composite endpoint of CV death, MI or ischaemic stroke Study population Patients with Type 2 diabetes aged ≥ 40 years with established CVD or ≥ 2 risk factors Statistical power Event driven: 1390 events / 99% power for non-inferiority, 85% power for superiority (HR=0. 85) Cardiac failure: Experience in NYHA Class I–II is limited, and there is no experience in clinical studies with FORXIGA in NYHA Class III–IV. FORXIGA is not recommended for use in patients receiving loop diuretics or who are volume depleted, e. g. due to acute illness (such as gastrointestinal illness). Caution should be exercised in patients for whom a dapagliflozin-induced drop in blood pressure could pose a risk, such as patients with known CVD, patients on antihypertensive therapy with a history of hypotension or elderly patients. CV, cardiovascular; CVD, cardiovascular disease; NYHA, New York Heart Association; SBP, systolic blood pressure. 1. Sonesson C, et al. Cardiovasc Diabetol 2016; 15: 37; 2. Cefalu WT, et al. Presented at the 75 th American Diabetes Association Scientific Sessions, Boston, USA. 5– 9 June 2015; Abstract 2611; 3. DECLARE. Clinicaltrials. gov. NCT 01730534. Available at: https: //clinicaltrials. gov/ct 2/show/NCT 01730534? term=DECLARE&rank=2. Last accessed April 2016. DECLARE. Clinicaltrials. gov. NCT 01730534. Available at: https: //clinicaltrials. gov/ct 2/show/NCT 01730534? term=DECLARE&rank=2. Last accessed April 2015

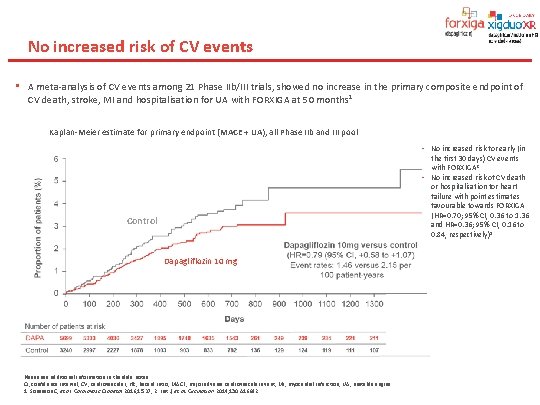
No increased risk of CV events • A meta-analysis of CV events among 21 Phase IIb/III trials, showed no increase in the primary composite endpoint of CV death, stroke, MI and hospitalisation for UA with FORXIGA at 50 months 1 Kaplan-Meier estimate for primary endpoint (MACE + UA), all Phase IIb and III pool • No increased risk for early (in the first 30 days) CV events with FORXIGA 2 • No increased risk of CV death or hospitalisation for heart failure with point estimates favourable towards FORXIGA (HR=0. 70; 95% CI, 0. 36 to 1. 36 and HR=0. 36; 95% CI, 0. 16 to 0. 84, respectively) 1 Control Dapagliflozin 10 mg Please see additional information in the slide notes. CI, confidence interval, CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event; MI, myocardial infarction; UA, unstable angina. 1. Sonesson C, et al. Cardiovasc Diabetol 2016; 15: 37; 2. List J, et al. Circulation 2014; 130: A 16682.

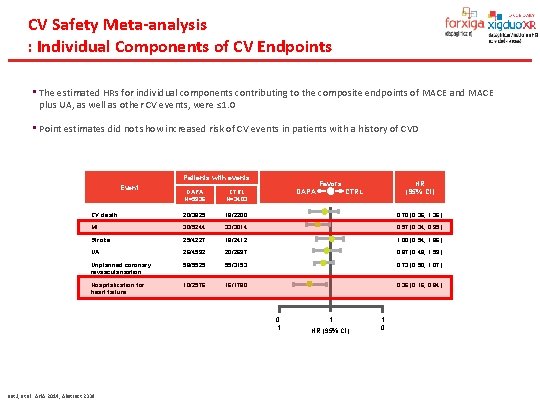
CV Safety Meta-analysis : Individual Components of CV Endpoints • The estimated HRs for individual components contributing to the composite endpoints of MACE and MACE plus UA, as well as other CV events, were ≤ 1. 0 • Point estimates did not show increased risk of CV events in patients with a history of CVD Patients with events Event Favors DAPA HR (95% Cl) CTRL DAPA N=5936 CTRL N=3403 CV death 20/3825 18/2200 0. 70 (0. 36, 1. 36) MI 30/5244 33/3014 0. 57 (0. 34, 0. 95) Stroke 25/4227 18/2412 1. 00 (0. 54, 1. 86) UA 26/4592 20/2697 0. 87 (0. 48, 1. 59) Unplanned coronary revascularisation 58/5525 55/3153 0. 73 (0. 50, 1. 07) Hospitalization for heart failure 10/2576 16/1780 0. 36 (0. 16, 0. 84) 0. 1 List J, et al. AHA 2014, Abstract 2339 1 HR (95% Cl) 1 0

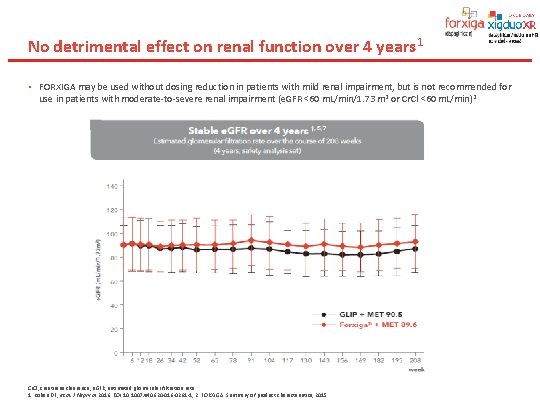
No detrimental effect on renal function over 4 years 1 • FORXIGA may be used without dosing reduction in patients with mild renal impairment, but is not recommended for use in patients with moderate-to-severe renal impairment (e. GFR <60 m. L/min/1. 73 m 2 or Cr. Cl <60 m. L/min)2 Cr. Cl, creatinine clearance; e. GFR, estimated glomerular filtration rate. 1. Kohan DE, et al. J Nephrol 2016. DOI 10. 1007/s 40620 -016 -0261 -1; 2. FORXIGA. Summary of product characteristics, 2015.

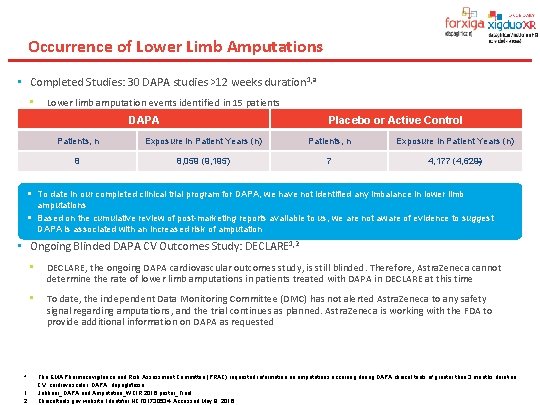
Occurrence of Lower Limb Amputations • Completed Studies: 30 DAPA studies >12 weeks duration 1, a • Lower limb amputation events identified in 15 patients DAPA Placebo or Active Control Patients, n Exposure in Patient Years (n) 8 8, 059 (9, 195) 7 4, 177 (4, 629) § To date in our completed clinical trial program for DAPA, we have not identified any imbalance in lower limb amputations § Based on the cumulative review of post-marketing reports available to us, we are not aware of evidence to suggest DAPA is associated with an increased risk of amputation • Ongoing Blinded DAPA CV Outcomes Study: DECLARE 1, 2 a 1. 2. • DECLARE, the ongoing DAPA cardiovascular outcomes study, is still blinded. Therefore, Astra. Zeneca cannot determine the rate of lower limb amputations in patients treated with DAPA in DECLARE at this time • To date, the independent Data Monitoring Committee (DMC) has not alerted Astra. Zeneca to any safety signal regarding amputations, and the trial continues as planned. Astra. Zeneca is working with the FDA to provide additional information on DAPA as requested The EMA Pharmacovigilance and Risk Assessment Committee (PRAC) requested information on amputations occurring during DAPA clinical trials of greater than 3 months duration. CV, cardiovascular; DAPA, dapagliflozin. Jabbour_DAPA and Amputation_WCIR 2016 poster_Final Clinicaltrials. gov website. Identifier NCT 01730534. Accessed May 9, 2016.

Patient Group Profile

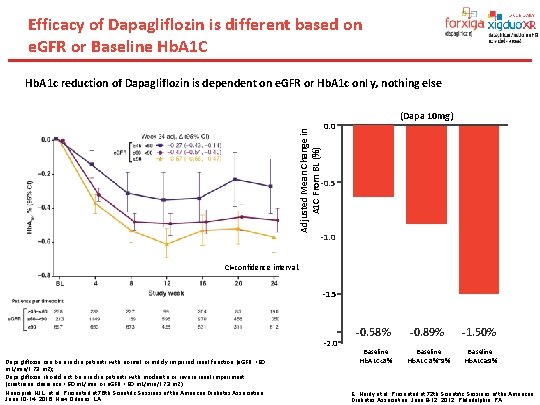
Efficacy of Dapagliflozin is different based on e. GFR or Baseline Hb. A 1 C Adjusted Mean Change in A 1 C From BL (%) Hb. A 1 c reduction of Dapagliflozin is dependent on e. GFR or Hb. A 1 c onl y, nothing else (Dapa 10 mg) 0. 0 -0. 5 -0. 7%† -1. 0 CI=confidence interval. -1. 5 -2. 0 Dapagliflozin can be used in patients with normal or mildly impaired renal function (e. GFR >60 m. L/min/1. 73 m 2); Dapagliflozin should not be used in patients with moderate or severe renal impairment (creatinine clearance <60 m. L/min or e. GFR <60 m. L/min/1. 73 m 2) Heerspink HJL, et al. Presented at 76 th Scientific Sessions of the American Diabetes Association, June 10 -14, 2016; New Orleans, LA. -0. 58% Baseline Hb. A 1 C<8% -0. 89% -1. 50% Baseline Hb. A 1 C 8%~9% Baseline Hb. A 1 C≥ 9% E, Hardy et al. Presented at 72 th Scientific Sessions of the American Diabetes Association, June 8 -12, 2012; Philadelphia, PA.

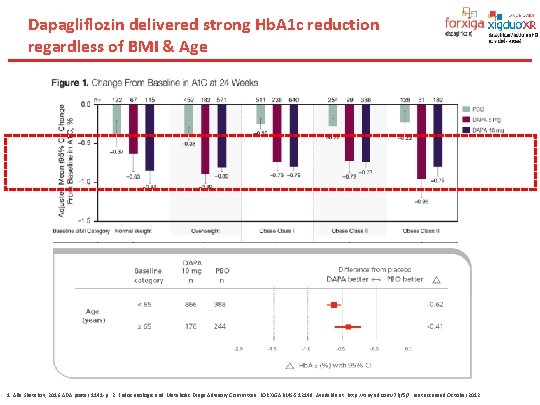
Dapagliflozin delivered strong Hb. A 1 c reduction regardless of BMI & Age 1. Alla Shatskov, 2016 ADA poster 1141 -p. 2. Endocrinologic and Metabolic Drugs Advisory Committee: FORXIGABMS-512148. Available at: http: //tinyurl. com/7 kjf 5 j 7. Last accessed October 2012

Dapagliflozin can make diverse patient groups smile

Summary & Key Takeaways • Paradigm shift towards EARLY USE of SGLT-2 i • SGLT-2 i as an effective treatment option for patients who are inadequately controlled with metformin, even before DPP-4 i or other OADs • SGLT-2 i provides stronger Hb. A 1 c reduction than DPP-4 i and additional benefits of weight loss & BP reduction • Dapagliflozin delivered strong Hb. A 1 C Reduction regardless of BMI and Age 1. 2. 3. 4. * Henry RR et al. Int J Clin Pract. 2012; 66: 446 -456. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581– 90; 2. Nauck MA, et al. Diabetes Care 2011; 34: 2015– 22. Sonesson C, et al. Cardiovasc Diabetol 2016; 15: 37; 2. List J, et al. Circulation 2014; 130: A 16682 Inzucchi SE, et al. Diabetes Care 2015; 38: 140 -9, 한국 식약처 허가기준 (2015. 5. 29) 5. Bailey CJ, et al. Lancet 2010; 37: 2223 -33. 6. Bailey CJ, et al. BMC Med 2013; 11: 43. 7. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581 -90, 8. FORXIGA®. Summary of product characteriastics, 2016. 9. Gaber AJ, et al Endocr Pract 2016; 22: 84 -113.

Thank you

Back up

Asian Patients with Metformin failure

Asian patients with T 2 DM after metformin failure : Study design Wenying Yang, et al. 74 sh. ADA Scientific Sessions, San Francisco, 13– 17 June 2014[Poster 1076 -P].

Asian patients with T 2 DM after metformin failure : Demographics Number of patients Age, years Sex, n(%) Male Female Race, n(%) Asian Indian Chinese Korean Hb. A 1 c, % FPG, mg/d. L PPG, mg/d. L Duration of T 2 DM, Years Weight, kg BMI, kg/m 2 Seated SBP, mm. Hg Seated DBP, mm. Hg Placebo DAPA 5 mg DAPA 10 mg 145 53. 5± 9. 2 147 53. 1± 9. 1 152 54. 6± 9. 5 86 (59. 3) 59(40. 7) 67 (45. 6) 80 (54. 4) 88 (57. 9) 64 (42. 1) 10 (6. 9) 126 (86. 9) 9 (6. 2) 8. 13± 0. 85 165. 5± 44. 8 254. 9± 61. 8 5. 3± 4. 4 70. 9± 11. 4 25. 7± 2. 9 126. 3± 13. 6 78. 3± 8. 5 11 (7. 5) 127 (86. 4) 9 (6. 1) 8. 09± 0. 72 161. 8± 40. 0 253. 5± 52. 1 4. 2± 3. 8 70. 8± 12. 2 26. 4± 3. 5 129. 1± 14. 4 78. 6± 8. 8 13 (8. 6) 129 (84. 9) 10 (6. 6) 8. 17± 0. 84 161. 6± 40. 0 254. 1± 62. 5 5. 3± 4. 6 71. 4± 12. 0 26. 2± 3. 5 127. 0± 13. 7 77. 6± 8. 7 Wenying Yang, et al. 74 sh. ADA Scientific Sessions, San Francisco, 13– 17 June 2014[Poster 1076 -P].

Asian patients with T 2 DM after metformin failure : Hb. A 1 c PBO Baseline = 8. 13% DAPA 5 mg Baseline = 8. 09% DAPA 10 mg Baseline = 8. 17% 0. 0 Week 24 value Change in Hb. A 1 c (%) * -0. 23 (-. 35, -0. 11) -0. 4 -0. 6 -0. 82 (0. 94, -0. 70) -0. 85 (0. 96, -0. 73) -0. 8 -1. 0 -1. 2 0 4 8 12 16 Study week Data are adjusted mean change from ANCOVA (LOCF) and exclude data after rescue therapy. ANCOVA, analysis of covariance; LOCF, last observation carried forward Wenying Yang, et al. 74 sh. ADA Scientific Sessions , San Francisco, 13– 17 June 2014[Poster 1076 -P]. 20 24

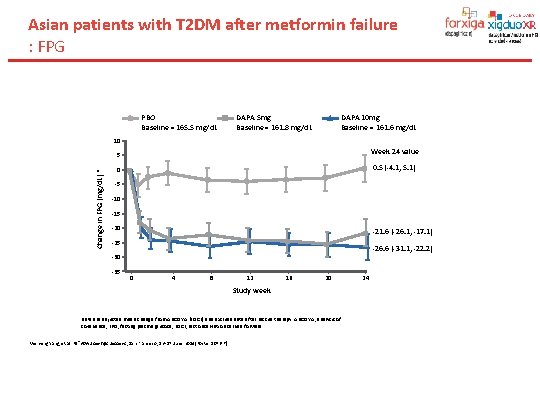
Asian patients with T 2 DM after metformin failure : FPG PBO Baseline = 165. 5 mg/d. L DAPA 5 mg Baseline = 161. 8 mg/d. L DAPA 10 mg Baseline = 161. 6 mg/d. L Change in FPG (mg/d. L) * 10 5 Week 24 value 0 0. 5 (-4. 1, 5. 1) -5 -10 -15 -20 -21. 6 (-26. 1, -17. 1) -25 -26. 6 (-31. 1, -22. 2) -30 -35 0 4 8 12 16 20 Study week Data are adjusted mean change from ANCOVA (LOCF) and exclude data after rescue therapy. ANCOVA, analysis of covariance; FPG, fasting plasma glucose; LOCF, last observation carried forward Wenying Yang, et al. 74 sh. ADA Scientific Sessions , San Francisco, 13– 17 June 2014[Poster 1076 -P]. 24

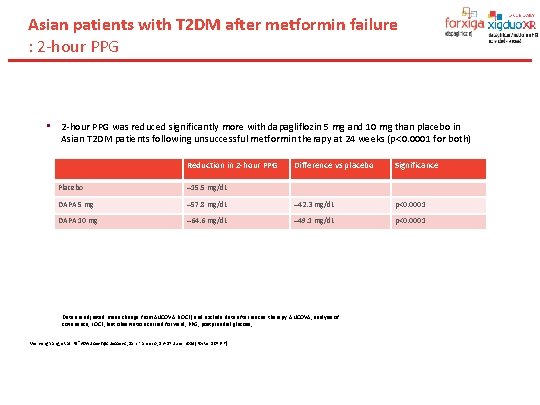
Asian patients with T 2 DM after metformin failure : 2 -hour PPG • 2 -hour PPG was reduced significantly more with dapagliflozin 5 mg and 10 mg than placebo in Asian T 2 DM patients following unsuccessful metformin therapy at 24 weeks (p 0. 0001 for both) Reduction in 2 -hour PPG Difference vs placebo Significance Placebo 15. 5 mg/d. L DAPA 5 mg 57. 8 mg/d. L 42. 3 mg/d. L p 0. 0001 DAPA 10 mg 64. 6 mg/d. L 49. 1 mg/d. L p 0. 0001 Data are adjusted mean change from ANCOVA (LOCF) and exclude data after rescue therapy. ANCOVA, analysis of covariance; LOCF, last observation carried forward; PPG, postprandial glucose; Wenying Yang, et al. 74 sh. ADA Scientific Sessions , San Francisco, 13– 17 June 2014[Poster 1076 -P].

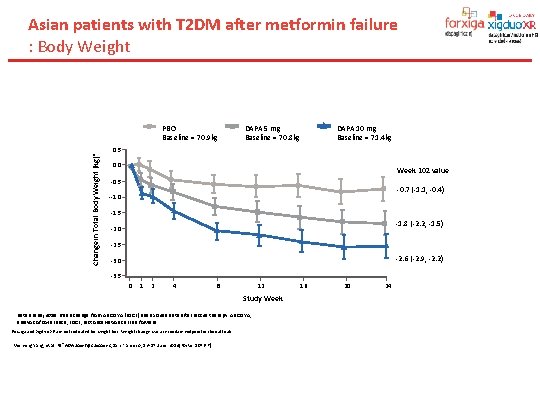
Asian patients with T 2 DM after metformin failure : Body Weight Change in Total Body Weight (kg)* PBO Baseline = 70. 9 kg DAPA 5 mg Baseline = 70. 8 kg DAPA 10 mg Baseline = 71. 4 kg 0. 5 0. 0 Week 102 value -0. 5 -0. 7 (-1. 1, -0. 4) --1. 0 -1. 5 -1. 8 (-2. 2, -1. 5) -2. 0 -2. 5 -2. 6 (-2. 9, -2. 2) -3. 0 -3. 5 0 1 2 4 8 12 Study Week Data are adjusted mean change from ANCOVA (LOCF) and exclude data after rescue therapy. ANCOVA, analysis of covariance; LOCF, last observation carried forward Forxiga and Xigduo XR are not indicated for weight loss. Weight change was a secondary endpoint in clinical trials Wenying Yang, et al. 74 sh. ADA Scientific Sessions , San Francisco, 13– 17 June 2014[Poster 1076 -P]. 16 20 24

The Functions of the Kidney Promote Glucose Homeostasis in the Body Glucose input ~250 g/day: • • Glucose uptake ~250 g/day: Dietary intake ~180 g/day Glucose production ~70 g/day • – Gluconeogenesis (liver, kidney) – Glycogenolysis (liver) Brain ~125 g/day • Kidney ~25 g/day • Rest of the body ~100 g/day The kidney reabsorbs and recirculates glucose Glucose filtered ~180 g/day 1. 2. Glucose reabsorbed ~180 g/day Gerich JE. Diabet Med. 2010; 27: 136 -142. Wright EM et al. J Intern Med. 2007; 261: 32 -43. 62

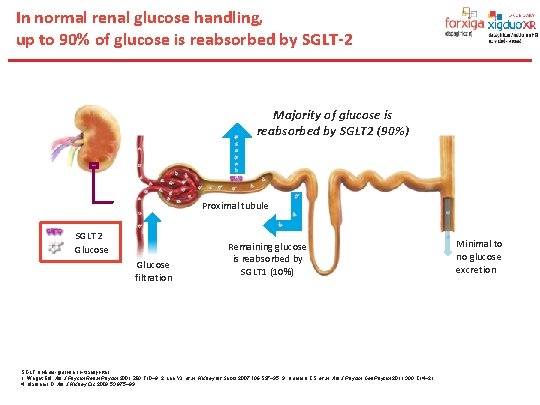
In normal renal glucose handling, up to 90% of glucose is reabsorbed by SGLT-2 Majority of glucose is reabsorbed by SGLT 2 (90%) Proximal tubule SGLT 2 Glucose filtration Remaining glucose is reabsorbed by SGLT 1 (10%) SGLT, sodium-glucose co-transporter. 1. Wright EM. Am J Physiol Renal Physiol 2001; 280: F 10– 8; 2. Lee YJ, et al. Kidney Int Suppl 2007; 106: S 27– 35; 3. Hummel CS, et al. Am J Physiol Cell Physiol 2011; 300: C 14– 21; 4. Marsenic O. Am J Kidney Dis 2009; 53: 875– 83. Minimal to no glucose excretion

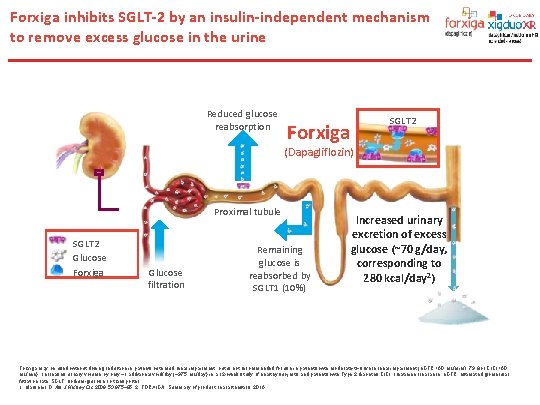
Forxiga inhibits SGLT-2 by an insulin-independent mechanism to remove excess glucose in the urine Reduced glucose reabsorption Forxiga SGLT 2 (Dapagliflozin) Proximal tubule SGLT 2 Glucose Forxiga Glucose filtration Remaining glucose is reabsorbed by SGLT 1 (10%) Increased urinary excretion of excess glucose (~70 g/day, corresponding to 280 kcal/day 2) 2 or Cr. Cl <60 Forxiga may be used without dosing reduction in patients with mild renal impairment, but is not recommended for use in patients with moderate-to-severe renal impairment (e. GFR <60 m. L/min/1. 73 m 1 Cr. Cl, creatinine clearance; e. GFR, estimated glomerular m. L/min). 1 Increases urinary volume by only ~1 additional void/day (~375 m. L/day) in a 12 -week study of healthy subjects and patients with Type 2 diabetes. filtration rate; SGLT, sodium-glucose co-transporter. 1. Marsenic O. Am J Kidney Dis 2009; 53: 875– 85; 2. FORXIGA. Summary of product characteristics, 2016.

Forxiga inhibits SGLT-2 by an insulin-independent mechanism to remove excess glucose in the urine Reduced glucose reabsorption Forxiga SGLT 2 (Dapagliflozin) Proximal tubule SGLT 2 Glucose Forxiga Glucose filtration Remaining glucose is reabsorbed by SGLT 1 (10%) Increased urinary excretion of excess glucose (~70 g/day, corresponding to 280 kcal/day 2) 2 or Cr. Cl <60 Forxiga may be used without dosing reduction in patients with mild renal impairment, but is not recommended for use in patients with moderate-to-severe renal impairment (e. GFR <60 m. L/min/1. 73 m 1 Cr. Cl, creatinine clearance; e. GFR, estimated glomerular m. L/min). 1 Increases urinary volume by only ~1 additional void/day (~375 m. L/day) in a 12 -week study of healthy subjects and patients with Type 2 diabetes. filtration rate; SGLT, sodium-glucose co-transporter. 1. Marsenic O. Am J Kidney Dis 2009; 53: 875– 85; 2. FORXIGA. Summary of product characteristics, 2016.

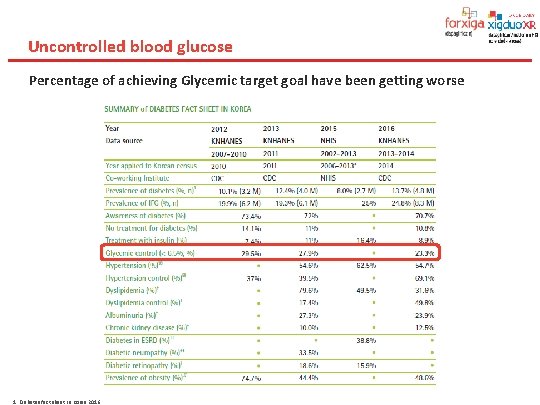
Uncontrolled blood glucose Percentage of achieving Glycemic target goal have been getting worse 1. Diabetes fact sheet in Korea 2016

Summary & Key Takeaways • XIGDUO XR as the *FIRST and ONLY combination drug of SGLT-2 i and Metformin XR in Korea • • • 1. 2. 3. 4. * Fast glucose lowering effect as early as 4 weeks 2% Hb. A 1 C reduction at 24 weeks Sustained efficacy over 4 years vs. SU Additional benefit of weight loss and blood pressure reduction No increased risk of CV events Henry RR et al. Int J Clin Pract. 2012; 66: 446 -456. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581– 90; 2. Nauck MA, et al. Diabetes Care 2011; 34: 2015– 22. Sonesson C, et al. Cardiovasc Diabetol 2016; 15: 37; 2. List J, et al. Circulation 2014; 130: A 16682 Inzucchi SE, et al. Diabetes Care 2015; 38: 140 -9, 한국 식약처 허가기준 (2015. 5. 29) 5. Bailey CJ, et al. Lancet 2010; 37: 2223 -33. 6. Bailey CJ, et al. BMC Med 2013; 11: 43. 7. Del Prato S, et al. Diabetes Obes Metab 2015; 17: 581 -90, 8. FORXIGA®. Summary of product characteriastics, 2016. 9. Gaber AJ, et al Endocr Pract 2016; 22: 84 -113.

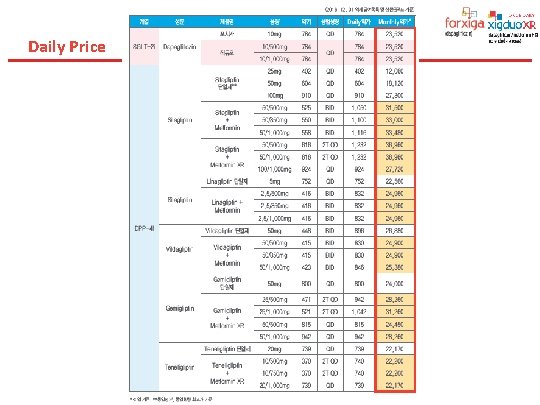
Daily Price
 Unmet needs in severe asthma
Unmet needs in severe asthma Unmet financial need
Unmet financial need Primary needs and secondary needs
Primary needs and secondary needs Satisfaction
Satisfaction Simple claustral complex
Simple claustral complex Strategic gender needs and practical gender needs
Strategic gender needs and practical gender needs Need analysis definition
Need analysis definition Colonialism and development: korea, taiwan, and kwantung
Colonialism and development: korea, taiwan, and kwantung How are the physical features of japan and korea similar?
How are the physical features of japan and korea similar? Used in both the folk and classical music of korea
Used in both the folk and classical music of korea Spread of china's literature to heian japan and korea
Spread of china's literature to heian japan and korea Chapter 12 section 5 kingdoms of southeast asia and korea
Chapter 12 section 5 kingdoms of southeast asia and korea Korea occupational safety
Korea occupational safety Why did japan annex korea
Why did japan annex korea Schaeffler korea
Schaeffler korea Lorna simpson
Lorna simpson Why did korea split
Why did korea split Religion in south korea
Religion in south korea When did korea split
When did korea split Goodhill korea centre pte ltd
Goodhill korea centre pte ltd Korean language letters
Korean language letters Korea hanyang
Korea hanyang Kupid korea university
Kupid korea university Korea-eu fta certificate of origin
Korea-eu fta certificate of origin What is the economic continuum
What is the economic continuum Dns server korea
Dns server korea Tanma korea
Tanma korea Pt duckil textile korea indonesia
Pt duckil textile korea indonesia Rebelyong nanking
Rebelyong nanking Dow chemical korea ltd
Dow chemical korea ltd Why is north korea switched off from globalisation
Why is north korea switched off from globalisation South korea map
South korea map G to g
G to g Sokbaji
Sokbaji Korea institute of sport science
Korea institute of sport science Korea meteorological administration
Korea meteorological administration Aaron fyke
Aaron fyke Korea housing finance corporation
Korea housing finance corporation Korea artifacts
Korea artifacts Matematika korea
Matematika korea Daihan labtech korea
Daihan labtech korea Uwayapply korea university
Uwayapply korea university Korean nazarene university
Korean nazarene university What is the weather like
What is the weather like Korea kosha
Korea kosha Neis korea
Neis korea Korea waste management
Korea waste management Ion vpn iptv
Ion vpn iptv Sinification of korea
Sinification of korea Foreign exchange transaction regulation korea
Foreign exchange transaction regulation korea Standard diagnostics inc
Standard diagnostics inc Lipton korea
Lipton korea Kosha regulation
Kosha regulation Korean war map activity pdf answers
Korean war map activity pdf answers Korea rail network authority
Korea rail network authority Kira south korea
Kira south korea Japans literacy rate
Japans literacy rate Donga university korea
Donga university korea English village korea
English village korea North korea genocide 1948-1994
North korea genocide 1948-1994 Cnf korea
Cnf korea South korea japan bridge
South korea japan bridge National atlas of korea
National atlas of korea Hangul origin
Hangul origin Ossc korea
Ossc korea Farm tg
Farm tg Korea
Korea New era korea
New era korea Ju bang kioh
Ju bang kioh