Unit The Atom and The Periodic Table Evolution

Unit: The Atom and The Periodic Table
Evolution of the Structure of an Atom

Dalton’s Atomic Theory • All elements are composed of indivisible particles called atoms • Atoms of the same element are identical. The atoms of any one element are different from those of any other element. • Atoms of different elements can physically mix together or chemically combine with one another in simple whole number ratios to form compounds. (law of definite composition) • Chemical rxns occur when atoms are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. (law of conservation of mass)
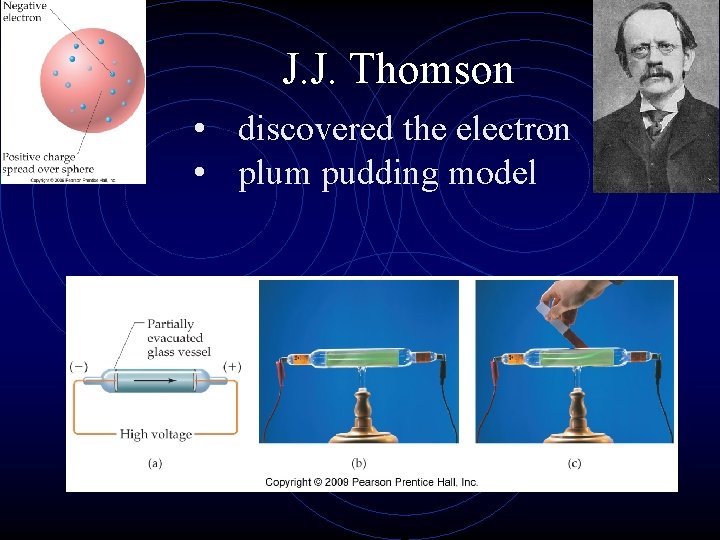
J. J. Thomson • discovered the electron • plum pudding model
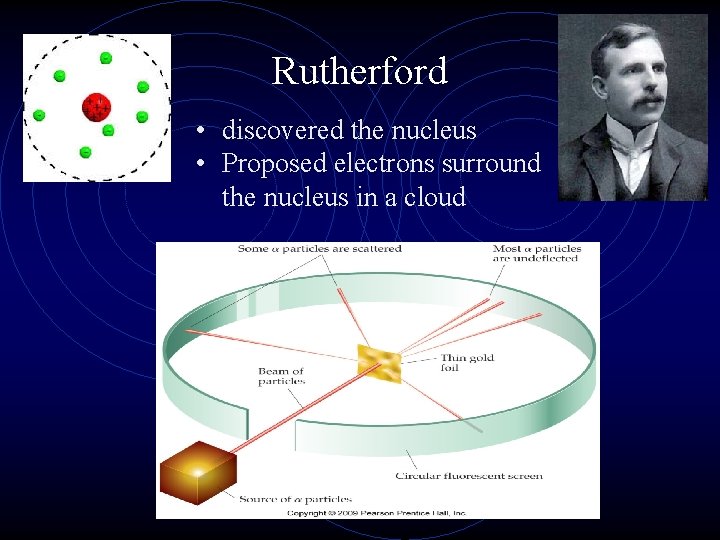
Rutherford • discovered the nucleus • Proposed electrons surround the nucleus in a cloud

The Atom Today • The atom is the smallest particle of an element that retains the properties of that element • Atoms can only be seen with proper instrumentation • Dalton’s Atomic Theory wasn’t completely correct • Atoms can be broken down into subatomic particles • Atoms of the same element are not identical
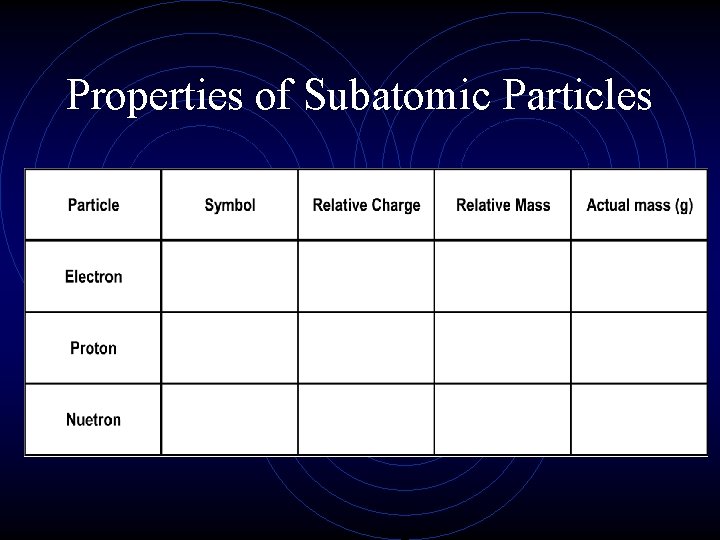
Properties of Subatomic Particles

Distinguishing Between Atoms

Atomic Number • Number of protons in the nucleus of an atom • Identifies the element • Elements are listed on periodic table according to atomic number
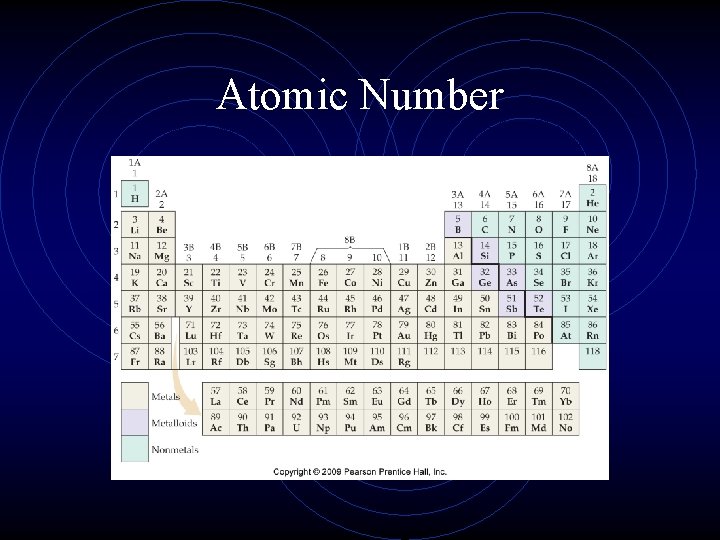
Atomic Number

Atomic Number

Atomic Number • atoms are electrically neutral • # protons = # electrons

Mass Number • total number of protons and neutrons in an atom • It is not found on the periodic table but can be estimated

Shorthand Notation

Fill in the following table

Isotopes • Atoms that have the same number of protons but different numbers of neutrons • Differ in their mass number

Isotopes • the existence of isotopes was not predicted by Dalton, who said atoms of the same element are the same

Isotopes • identified by their mass # , write the name of the element then a hyphen with the mass number

Isotopes

Uses of Isotopes • C-14 = archeological carbon dating • Am-241 = smoke alarms • I-131 = treating thyroid disorder • Co – 60 = cancer treatment

Atomic Mass (atomic weight) • weighted average mass of the atoms in naturally occurring samples of the element

Atomic Mass • reflects the mass and the relative abundance of isotopes as they occur in nature

Atomic Mass • To calculate the atomic mass of an element multiply the atomic mass of each isotope of the element by its relative abundance, then add the results atomic mass = mass 1 relabd. 1 + mass 2 rel. abd. 2+. . .

1. Calculate the % abundance of fictitious element Nv 2. If the mass of 293 Nv is 293. 15 amu (red)and that of 295 Nv is 295. 15 amu (blue), what is the atomic mass of Nv? .

Periodic Table • ________ was the first person to find a way to list elements in order of increasing atomic mass

Periodic Table • He constructed the first periodic table in 1869, an arrangement of the elements according to similarities in their properties.

Mendeleev’s Periodic Table

Periodic Table • In 1913, ______ determined the atomic number of atoms and arranged the modern periodic table according to atomic number.

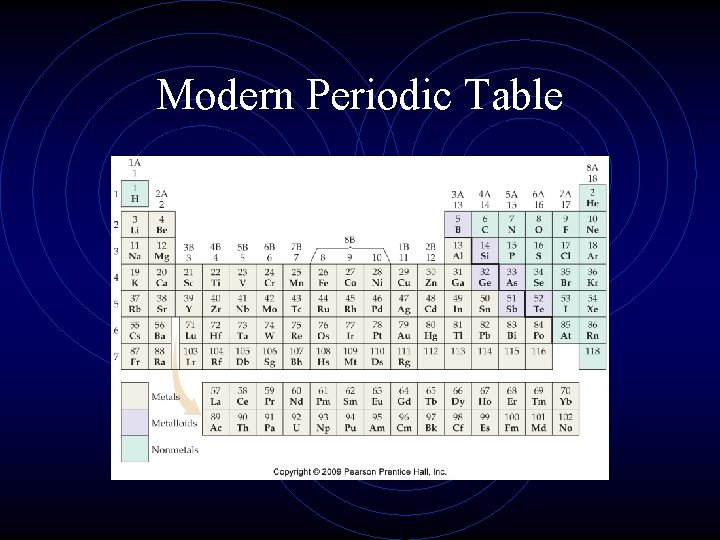
Modern Periodic Table

Modern Periodic Table • Each element is identified by its symbol placed in a square

Modern Periodic Table • The horizontal rows of the periodic table are called _____ • There are ___

Modern Periodic Table
Modern Periodic Table • Each vertical column is called a ____ or ____ • There are ____.
Modern Periodic Table
Periodic Table • A periodic law means that physical and chemical properties of elements are functions of their atomic numbers • Elements in the same group on the periodic table have similar properties

Modern Periodic Table • Group A elements are called the representative elements because they have a wide range of chemical and physical properties • Name the groups in Group A

Modern Periodic Table • Group B elements are called the _______ • The inner transition elements are the ______ and _____

Modern Periodic Table

Modern Periodic Table • The representative elements can be divided into three broad classes; Metals, Nonmetals, and Metalloids • What are some characteristics of each?
Modern Periodic Table

Niels Bohr • credited for a model of the atom that states that electrons are at specific distances from the nucleus of an atom
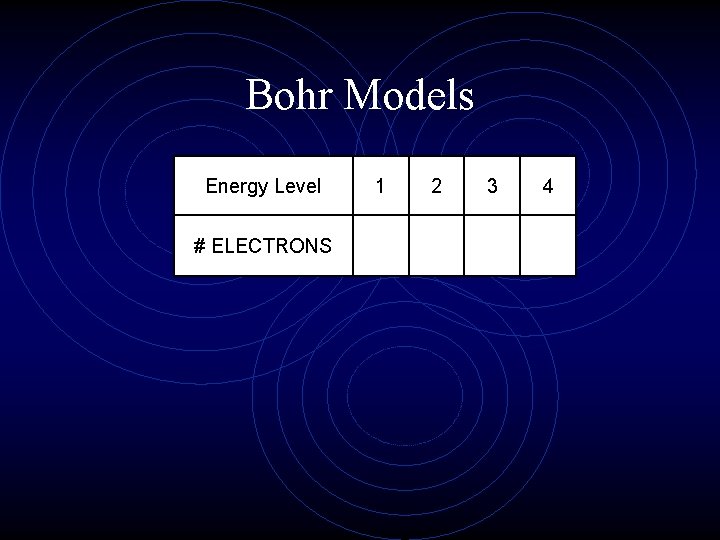
Bohr Models Energy Level 1 2 3 4 # ELECTRONS

Bohr Models Exception: Outermost energy level can only hold up to 8 e. You need to be able to diagram Bohr models for 1 -36; excluding transition elements

Bohr Models Do you notice any trends with the group or period of the periodic table?

Bohr Models Trends: 1. Group numbers (1 -8 A) predict valence electrons (electrons in the outmost level). 2. The period tells you how many energy levels you have in the atom.

- Slides: 46