Unit Outline Introduction Measures of Quantity Molar Mass




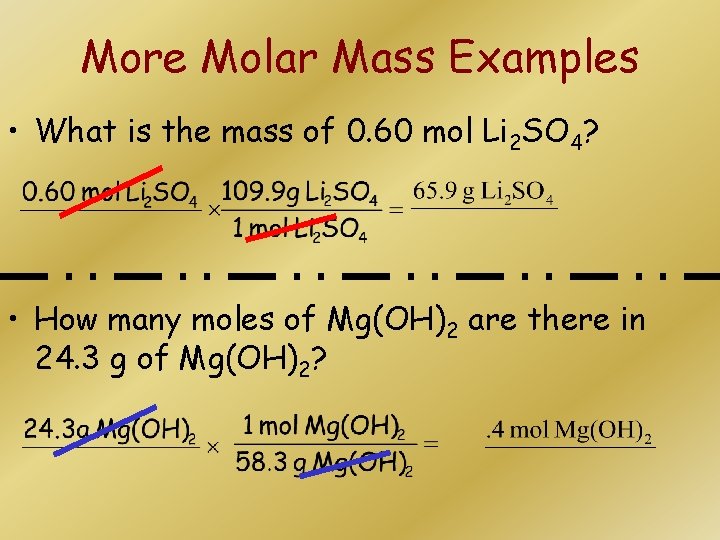




- Slides: 30


Unit Outline • Introduction : Measures of Quantity • Molar Mass • Molar Volume • Percent Composition • Finding Formulas

Measures of Quantity 1 dozen = 12 doughnuts EX 1 : How many doughnuts are in 4. 0 dozen? EX 2 : How many dozen are in 27 doughnuts?

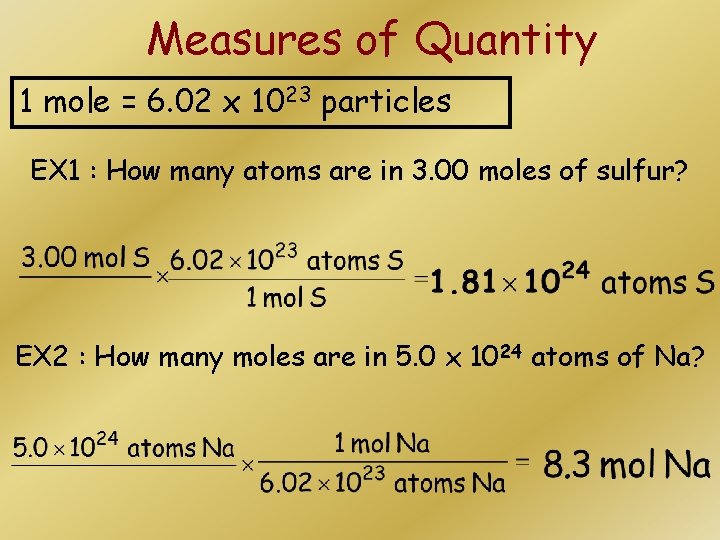
Measures of Quantity 1 mole = 6. 02 x 1023 particles EX 1 : How many atoms are in 3. 00 moles of sulfur? EX 2 : How many moles are in 5. 0 x 1024 atoms of Na?

Representative Particles • Atoms • Ions • Formula Units – Used for ionic compounds • Molecules – Used for covalent compounds

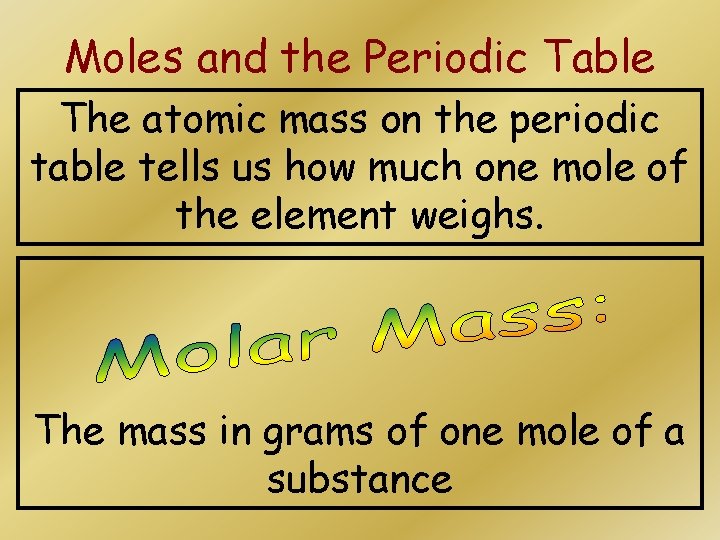
Moles and the Periodic Table The atomic mass on the periodic table tells us how much one mole of the element weighs. The mass in grams of one mole of a substance

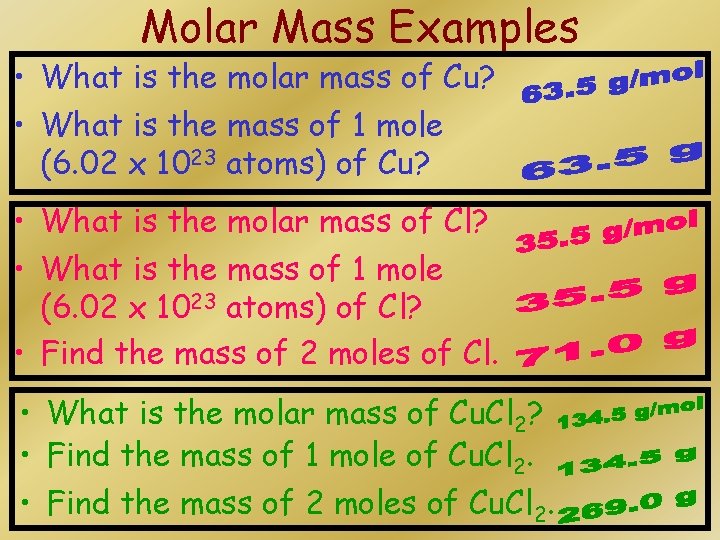
Molar Mass Examples • What is the molar mass of Cu? • What is the mass of 1 mole (6. 02 x 1023 atoms) of Cu? • What is the molar mass of Cl? • What is the mass of 1 mole (6. 02 x 1023 atoms) of Cl? • Find the mass of 2 moles of Cl. • What is the molar mass of Cu. Cl 2? • Find the mass of 1 mole of Cu. Cl 2. • Find the mass of 2 moles of Cu. Cl 2.
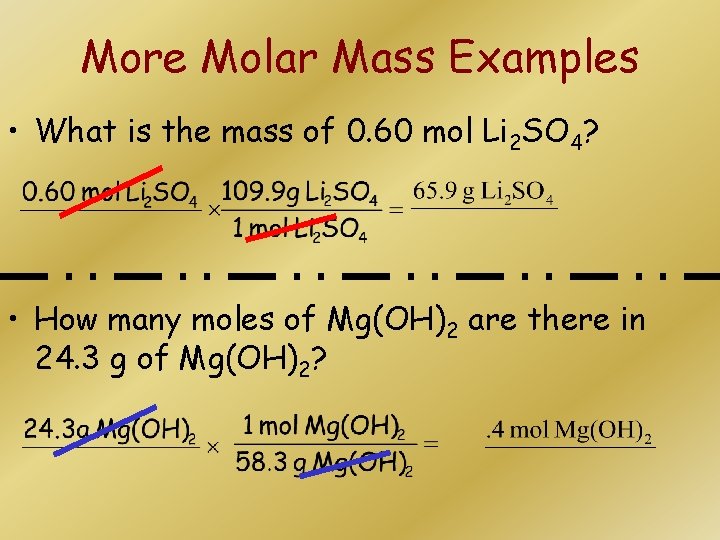
More Molar Mass Examples • What is the mass of 0. 60 mol Li 2 SO 4? • How many moles of Mg(OH)2 are there in 24. 3 g of Mg(OH)2?

Hints for Multi-Step Conversions ÑKEY #1 : Set up a roadmap. (airport example) ÑKEY #2 : Always start with what you are given. ÑKEY #3 : Always convert to moles first. ÑKEY #4 : Always include the units in your calculation. ÑKEY #5 : BE CAREFUL!

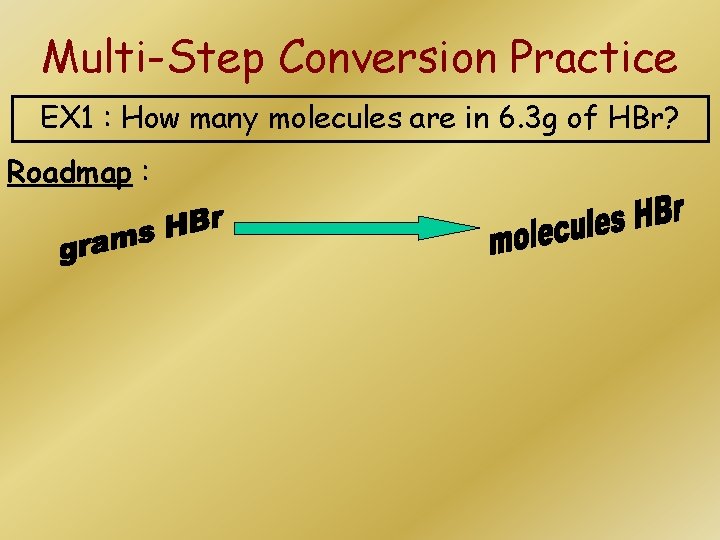
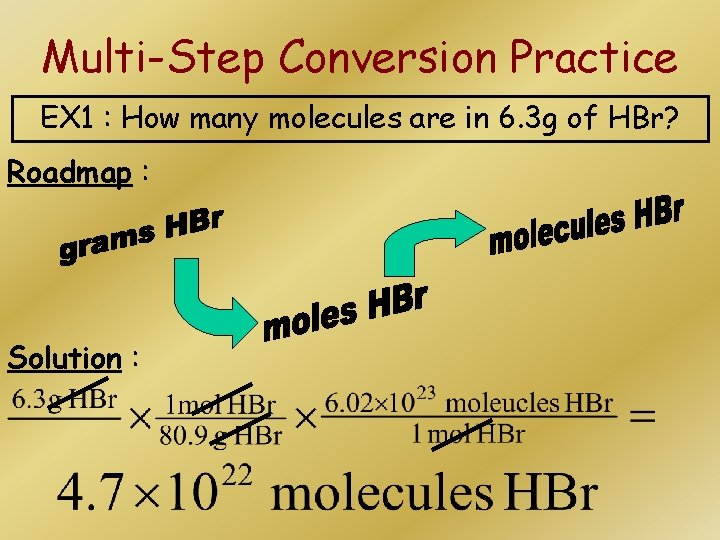
Multi-Step Conversion Practice EX 1 : How many molecules are in 6. 3 g of HBr? Roadmap :

Multi-Step Conversion Practice EX 1 : How many molecules are in 6. 3 g of HBr? Roadmap : Solution :

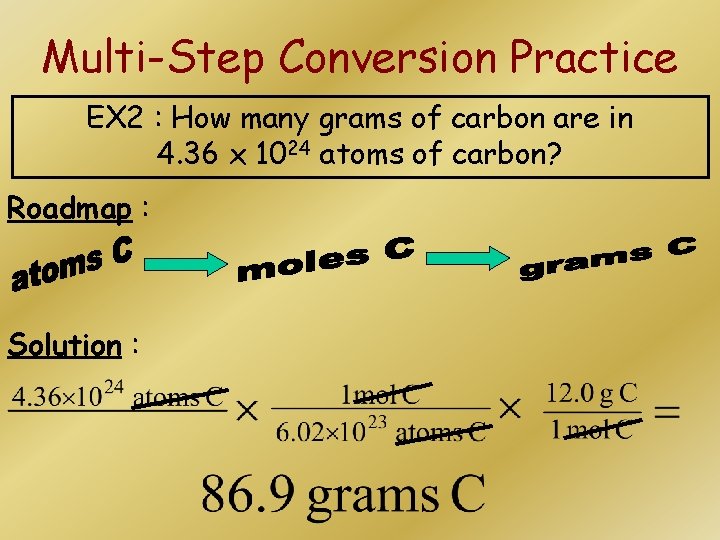
Multi-Step Conversion Practice EX 2 : How many grams of carbon are in 4. 36 x 1024 atoms of carbon? Roadmap : Solution :

Multi-Step Conversion Practice EX 3 : How many grams of magnesium sulfide are in 0. 32 moles of magnesium sulfide? Roadmap : Solution :

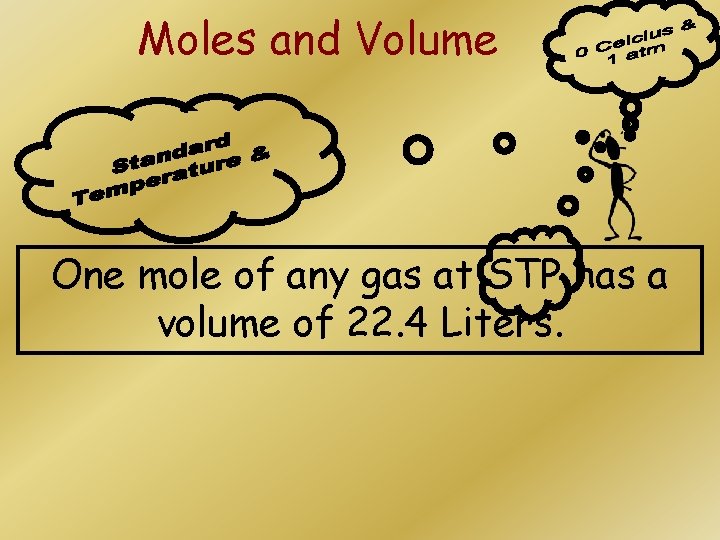
Moles and Volume One mole of any gas at STP has a volume of 22. 4 Liters.

Moles and Volume One mole of any gas at STP has a volume of 22. 4 Liters is often called the

• This works for any gas. • But gases have different masses right? How can they have the same volume? • Remember the Auditorium analogy.

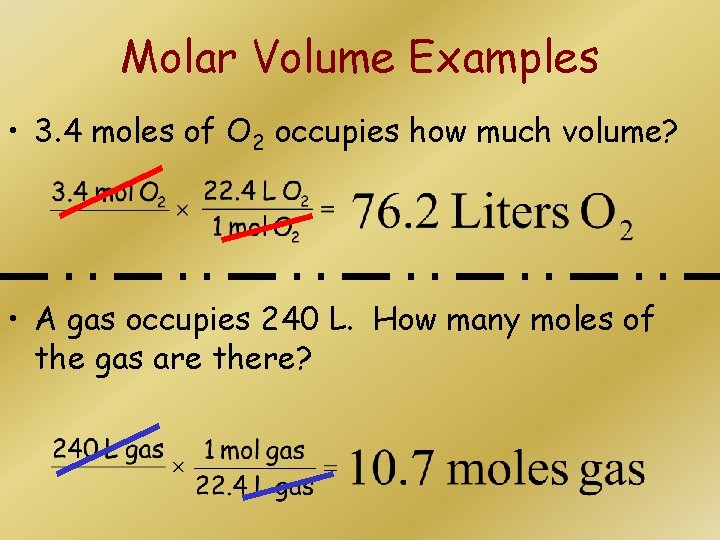
Molar Volume Examples • 3. 4 moles of O 2 occupies how much volume? • A gas occupies 240 L. How many moles of the gas are there?

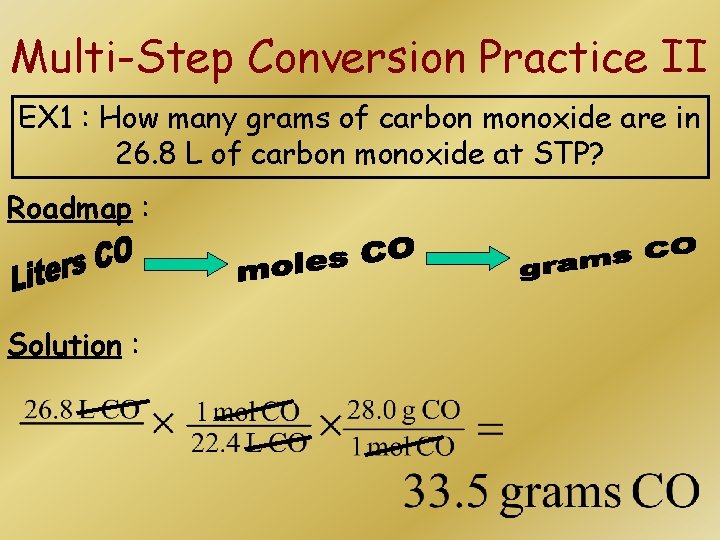
Multi-Step Conversion Practice II EX 1 : How many grams of carbon monoxide are in 26. 8 L of carbon monoxide at STP? Roadmap : Solution :

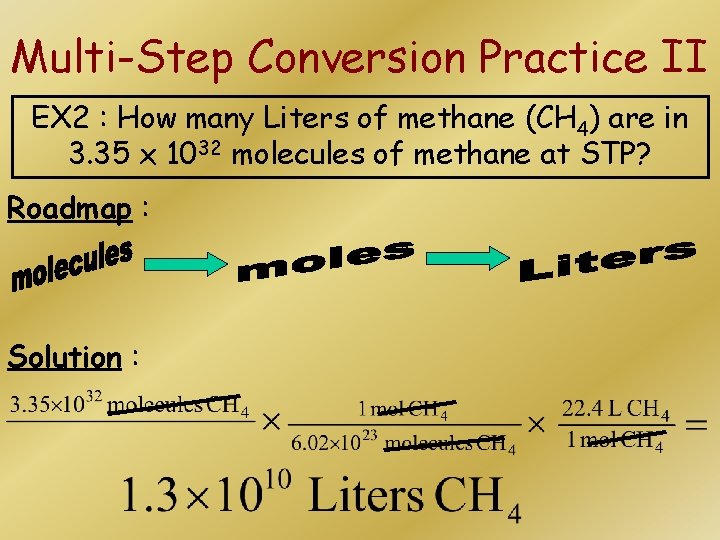
Multi-Step Conversion Practice II EX 2 : How many Liters of methane (CH 4) are in 3. 35 x 1032 molecules of methane at STP? Roadmap : Solution :

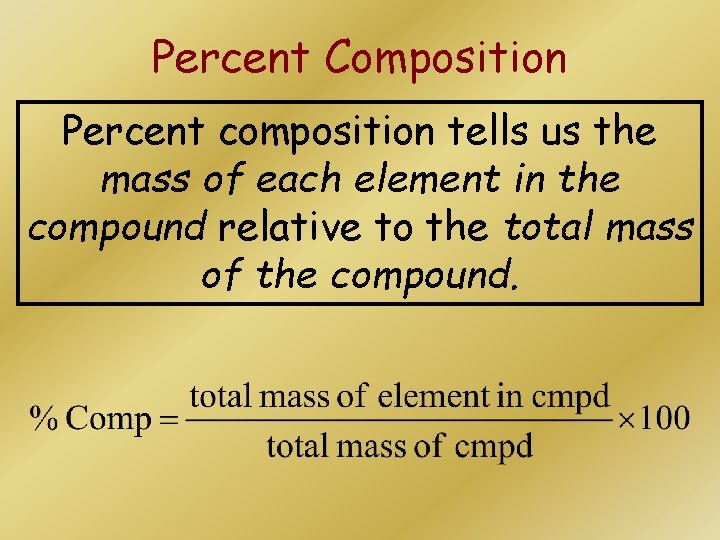
Percent Composition Percent composition tells us the mass of each element in the compound relative to the total mass of the compound.

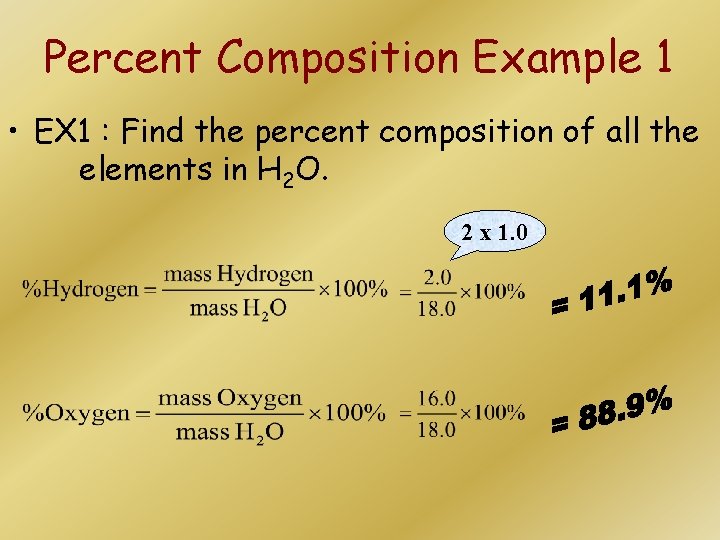
Percent Composition Example 1 • EX 1 : Find the percent composition of all the elements in H 2 O. 2 x 1. 0

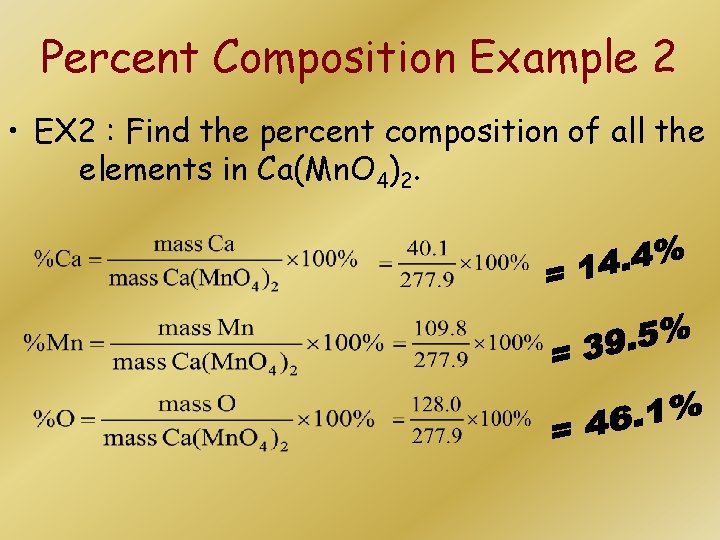
Percent Composition Example 2 • EX 2 : Find the percent composition of all the elements in Ca(Mn. O 4)2.

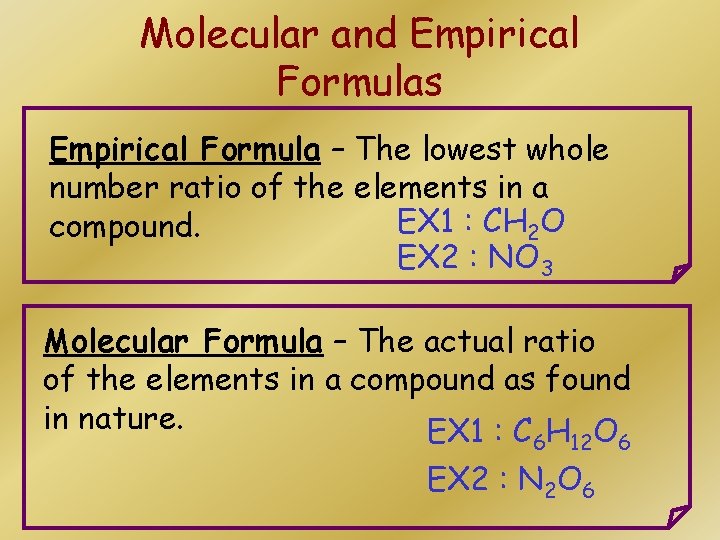
Molecular and Empirical Formulas Empirical Formula – The lowest whole number ratio of the elements in a EX 1 : CH 2 O compound. EX 2 : NO 3 Molecular Formula – The actual ratio of the elements in a compound as found in nature. EX 1 : C H O 6 12 EX 2 : N 2 O 6 6

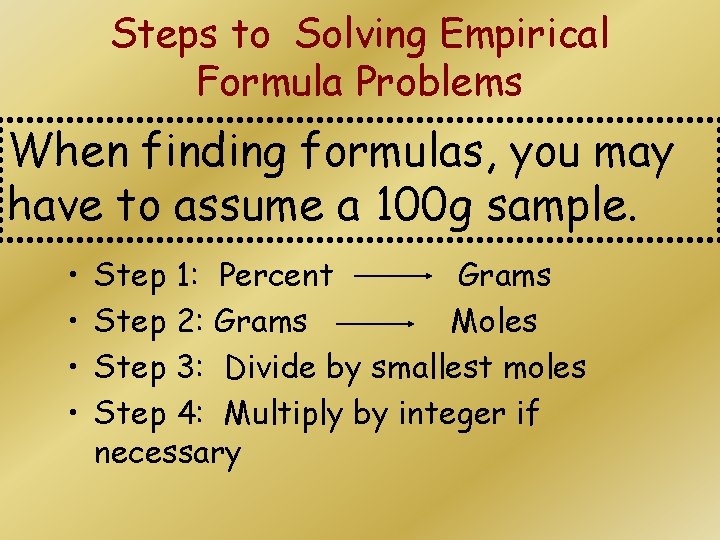
Steps to Solving Empirical Formula Problems When finding formulas, you may have to assume a 100 g sample. • • Step 1: Percent Grams Step 2: Grams Moles Step 3: Divide by smallest moles Step 4: Multiply by integer if necessary

Empirical Formula Example 1 EX 1 : A compound is found to be 27. 1% Na, 16. 5% N, and 56. 4% O. What is the empirical formula of the compound?

Empirical Formula Example 2: A compound is analyzed and found to contain 25. 8% N and 74. 2% O. What is the empirical formula of the compound?

Finding Molecular Formulas In order to find the molecular formula, you must have the following pieces of information: • The empirical formula • The molar mass of the molecule as found in nature.

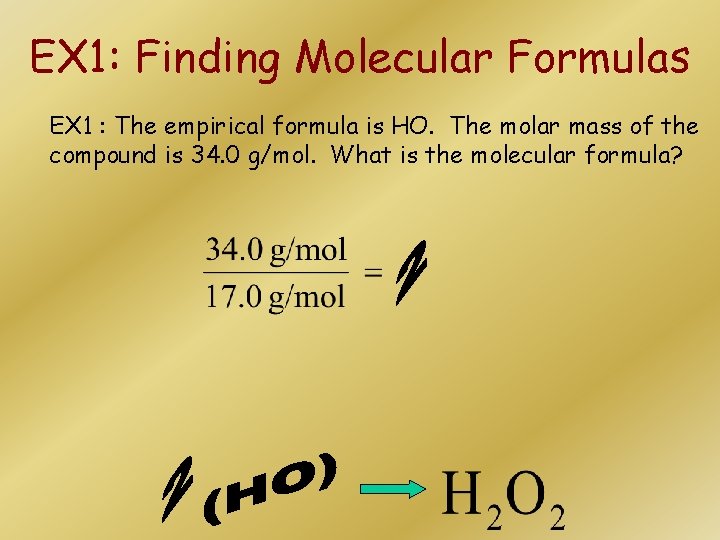
EX 1: Finding Molecular Formulas EX 1 : The empirical formula is HO. The molar mass of the compound is 34. 0 g/mol. What is the molecular formula?

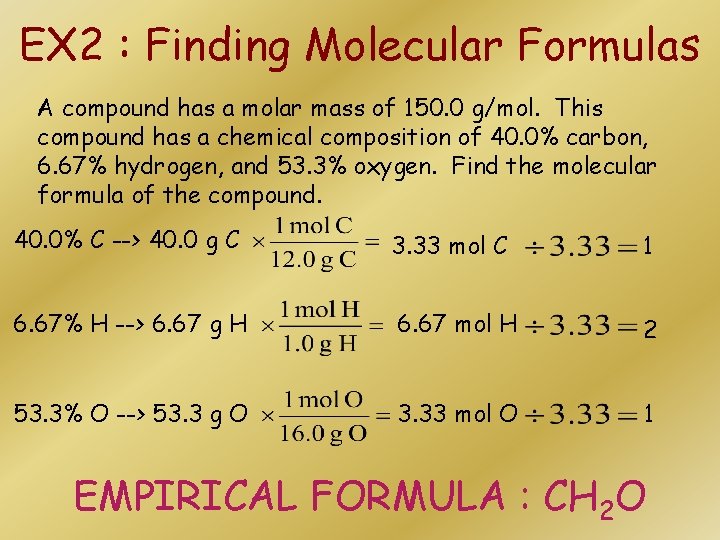
EX 2 : Finding Molecular Formulas A compound has a molar mass of 150. 0 g/mol. This compound has a chemical composition of 40. 0% carbon, 6. 67% hydrogen, and 53. 3% oxygen. Find the molecular formula of the compound. 40. 0% C --> 40. 0 g C 3. 33 mol C 1 6. 67% H --> 6. 67 g H 6. 67 mol H 2 53. 3% O --> 53. 3 g O 3. 33 mol O 1 EMPIRICAL FORMULA : CH 2 O

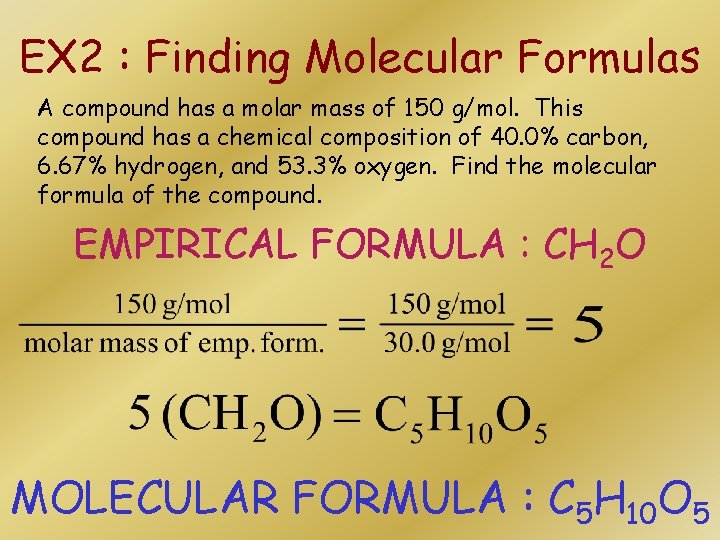
EX 2 : Finding Molecular Formulas A compound has a molar mass of 150 g/mol. This compound has a chemical composition of 40. 0% carbon, 6. 67% hydrogen, and 53. 3% oxygen. Find the molecular formula of the compound. EMPIRICAL FORMULA : CH 2 O MOLECULAR FORMULA : C 5 H 10 O 5