UNIT OPERATIONS IV HEAT TRANSFER CHE 3004 Lt










- Slides: 23

UNIT OPERATIONS IV HEAT TRANSFER CHE 3004 Lt. Col. G. Junior Virgo Ext. 3263

Learning Objectives At the end of this lesson you will: ◦ ◦ ◦ Know heat transfer basic concepts Understand the mechanism of conduction Understand the mechanism of convection Understand the mechanism of radiation Know the basic laws of heat transfer Know the application of various heat transfer equipment

Basic Concepts In our daily activities we often experience temperature changes of one form or another. ◦ Examples are refrigerators, ovens and stoves in our homes. automotive radiators, and electric fans. Car parked in the sun, walking into an air conditioned room

Basic Concepts Heat: Thermal energy in transition under the driving force of a temperature gradient Driving force: Bodies or systems at different reference points interacting or are made to interact causing flow in one direction.

HEAT TRANSFER Temperature gradient: from hot to cold or cold to hot The difference in temperature is the driving force that governs this phenomenon and the process is called HEAT TRANSFER.

HEAT TRANSFER The transfer of heat is integral to most chemical and engineering processes ◦ Heat absorbed or emitted ◦ Fluids heated or cooled ◦ Vapours condensed or solids melted Not only explains the mechanisms involved but also, the prediction of the applicable rates and magnitudes

HEAT TRANSFER All heat transfer must obey the first and second laws of thermodynamics ◦ 1 st Energy can neither be created nor destroyed but converted from one form to another ◦ 2 nd Heat always flows from higher to lower temperatures The transfer of heat at the desired rate is the fundamental principle upon which this entire course is based

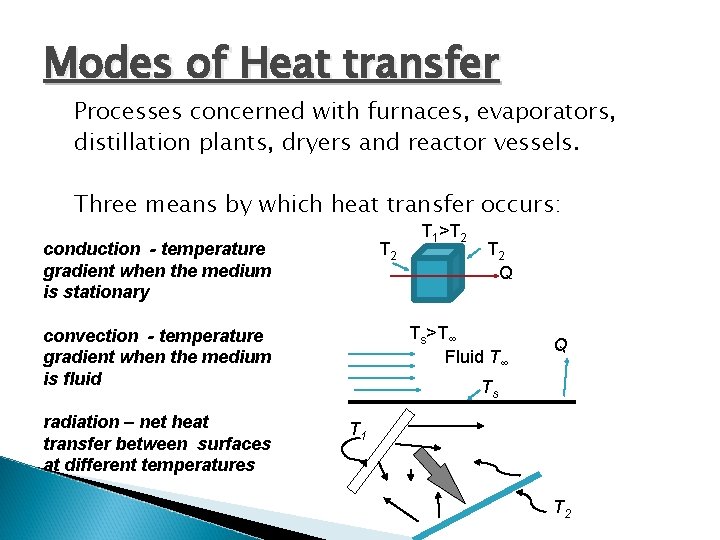
Modes of Heat transfer Processes concerned with furnaces, evaporators, distillation plants, dryers and reactor vessels. Three means by which heat transfer occurs: conduction - temperature gradient when the medium is stationary T 2 Q Ts>T∞ Fluid T∞ convection - temperature gradient when the medium is fluid radiation – net heat transfer between surfaces at different temperatures T 1>T 2 Q Ts T 1 T 2

Conduction Heat transfer at the intermolecular level of a substance Energetic interactions between adjacent particles. high energy is associated with high molecular activity hence heat applied to a particular point of a substance is passed on to other molecules in the immediate vicinity when they collide with each other.

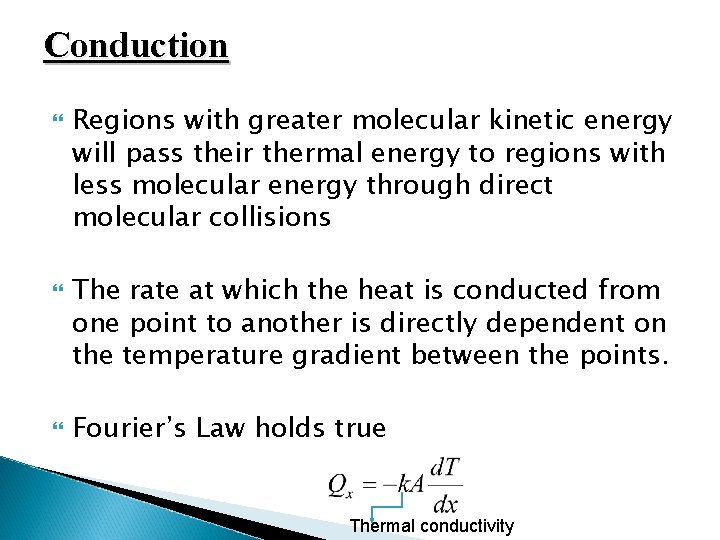
Conduction Regions with greater molecular kinetic energy will pass their thermal energy to regions with less molecular energy through direct molecular collisions The rate at which the heat is conducted from one point to another is directly dependent on the temperature gradient between the points. Fourier’s Law holds true Thermal conductivity

Convection Convective heat transfer ◦ The redistribution of energy due to: Conduction fluid motion combination of conduction within the boundary layer immediately adjacent to the heat source ◦ the motion of the bulk of the fluid brought about by the displacement of high-energy molecules due to fluid motion.

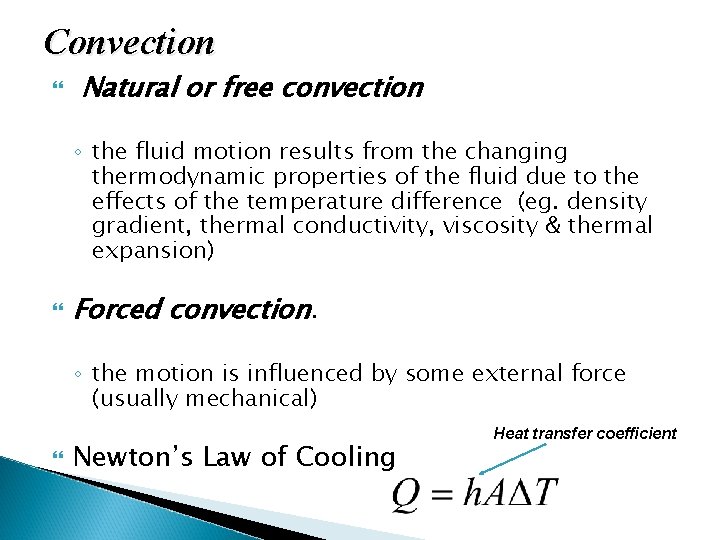
Convection Natural or free convection ◦ the fluid motion results from the changing thermodynamic properties of the fluid due to the effects of the temperature difference (eg. density gradient, thermal conductivity, viscosity & thermal expansion) Forced convection. ◦ the motion is influenced by some external force (usually mechanical) Newton’s Law of Cooling Heat transfer coefficient

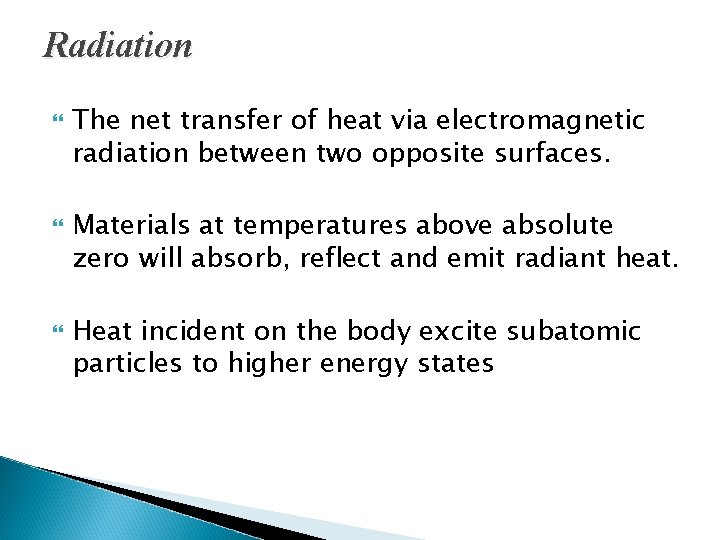
Radiation The net transfer of heat via electromagnetic radiation between two opposite surfaces. Materials at temperatures above absolute zero will absorb, reflect and emit radiant heat. Heat incident on the body excite subatomic particles to higher energy states

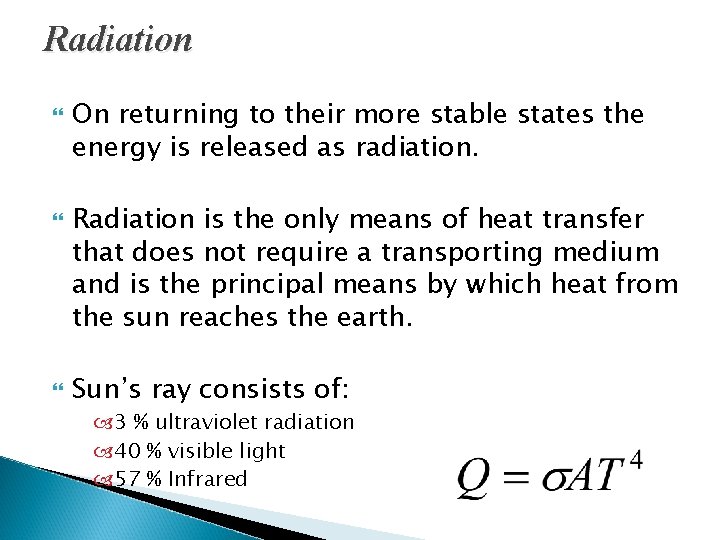
Radiation On returning to their more stable states the energy is released as radiation. Radiation is the only means of heat transfer that does not require a transporting medium and is the principal means by which heat from the sun reaches the earth. Sun’s ray consists of: 3 % ultraviolet radiation 40 % visible light 57 % Infrared

Heat Transfer Heat transfer applications are as diverse as the application of devices that ensure comfort and a high quality of life in our daily existence Usually a combination of one or more of the three modes mentioned above is involved. The human body is one of the most efficient heat transfer devices in operation in our daily lives.

Heat Transfer Organs involved are the lungs, skins and kidneys. We are totally dependent on electricity from power plants, refrigerators, stoves and air conditioning system. Heat exchangers are simply equipment consisting of two or more fluids at different temperatures that are physically separated by a wall to prevent fluid/material mixing.

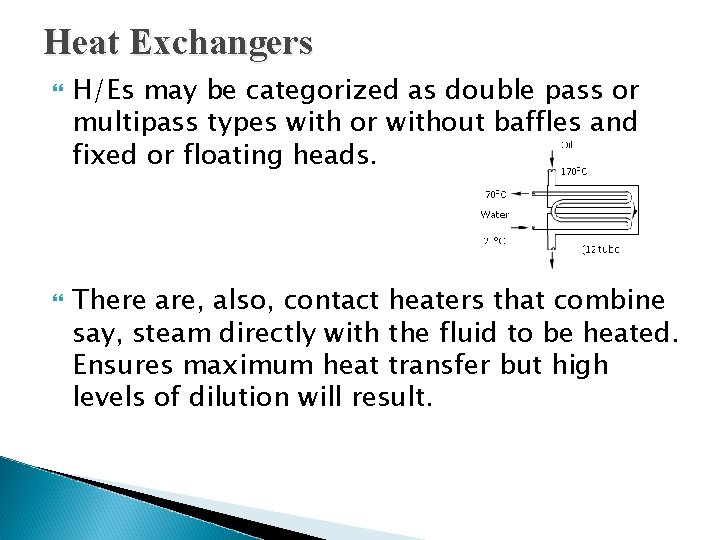
Heat Exchangers Depending on the inlet and outlet temperatures of the fluid of main concern a heat exchanger can be employed as either a “heater” or a “cooler”. The equipment design and orientation on a process plant depends largely on the nature of the fluids to be heated or cooled. Hence, we might employ double pipe (simplest form), shell and tube, spiral, and plate heat exchangers.

Heat Exchangers H/Es may be categorized as double pass or multipass types with or without baffles and fixed or floating heads. There are, also, contact heaters that combine say, steam directly with the fluid to be heated. Ensures maximum heat transfer but high levels of dilution will result.

Evaporative cooling A means of transferring heat between media and is the main unit operation of crystallization processes. Concentration of process streams is the major application of evaporation, which is usually accompanied by cooling by way of the latent heat of vapourization.

Equipment Heat transfer equipment in industrial plants varies from simple electrical fans for cooling to huge assemblies of pipes and conduits in arrangements called heat exchangers, Cooling fans are used in cooling towers to cool high volumes of heating fluids (usually water) that are used in heat exchangers to cool a particular product.

Equipment Cooling fans also used in electronic circuitry where the heating effects of electric current accounts for a very high heat per unit area in those continually decreasing components. Furnaces are equipment used to transfer heat directly to materials, for example in the calcining of ceramics, cement clinker or hydrated alumina in rotary kilns or fluid flash calciners.

Equipment Evaporators are vessels that concentrate solutions by boiling and evaporation. Variations include multiple effect evaporators connected in series to flash tanks in an intricate arrangement that ensures the recovery and reuse of flashed steam. Other equipment include heating elements used in water heaters, and solar panels

THE END THANK YOU