Unit One Notes 1 Unit One Two Classes

















- Slides: 42

Unit One

Notes #1 Unit One • • Two Classes of Elements Periodic Table Info? What Are Stable Elements? Oxidation/Reduction Oxidation Numbers Key Elements and Examples Pgs 158 -165

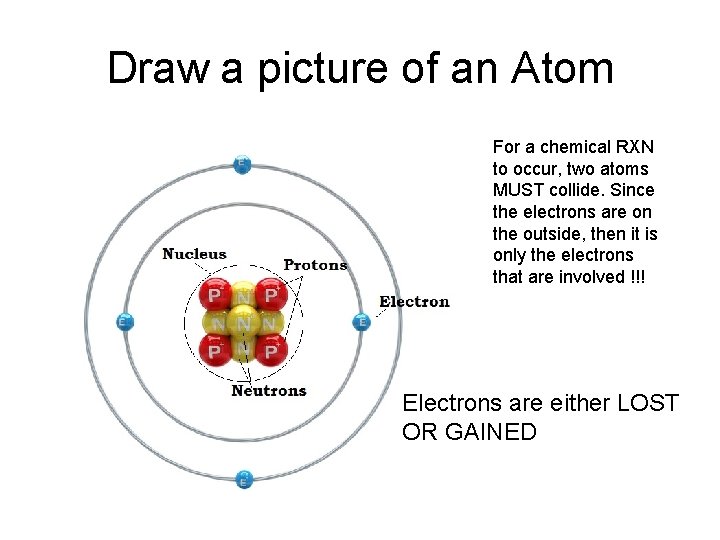
Draw a picture of an Atom For a chemical RXN to occur, two atoms MUST collide. Since the electrons are on the outside, then it is only the electrons that are involved !!! Electrons are either LOST OR GAINED

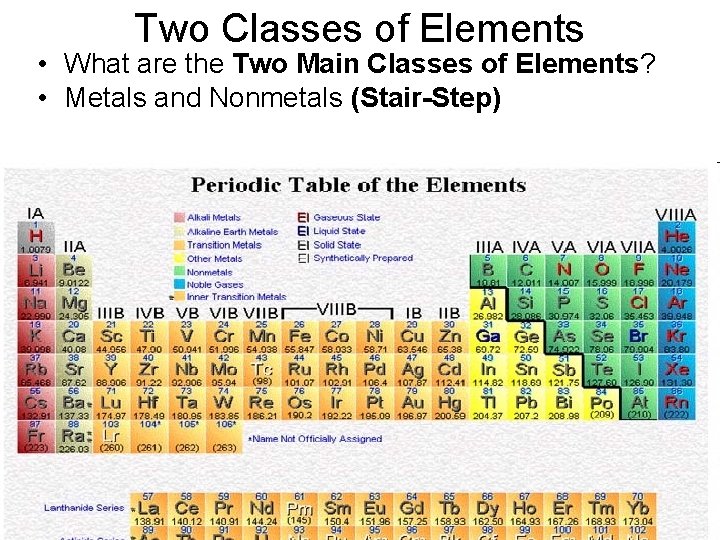
Two Classes of Elements • What are the Two Main Classes of Elements? • Metals and Nonmetals (Stair-Step)

What information doe the Periodic Table give us? Atomic Number # Protons = # of Electrons Mass Number = # P + # N Electron structure

So any Atom as no charge • The atomic number tells us the number of protons (+ charges) AND the number of electrons (- charges). • If you add all the charges up in an atom, they will equal ZERO. • An ATOM has NO NET Charge !!!

If we start with an Atom…. . • And it gains electrons, what happens to its’ charge? • It becomes negative • And it loses electrons, what happens to its’ chare? • It becomes positive. • If an atom becomes + or – we call it an ION

We can only change the # of electrons • If we were to try to change the number of protons, in the nucleus …. things have a tendency of going BOOM ! That is a nuclear reaction. So most chemical reactions involve the LOSS or GAIN of electrons • OXIDATION = Loss of electrons • REDUCTION = Gain of electrons

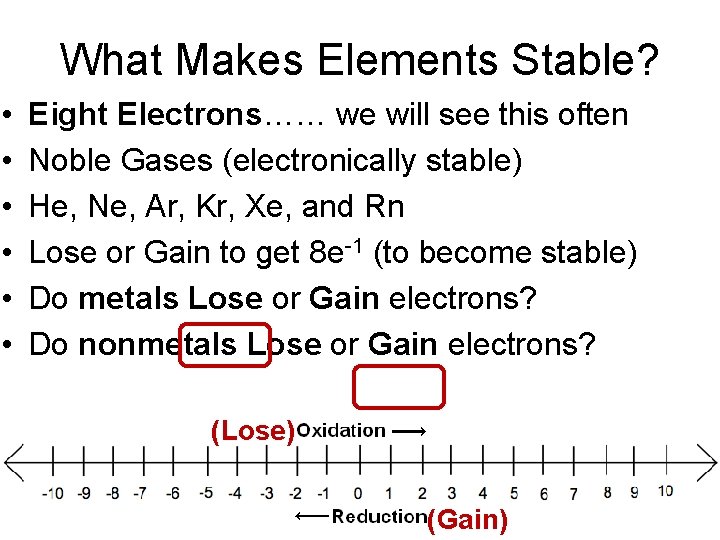
What Makes Elements Stable? • • • Eight Electrons…… we will see this often Noble Gases (electronically stable) He, Ne, Ar, Kr, Xe, and Rn Lose or Gain to get 8 e-1 (to become stable) Do metals Lose or Gain electrons? Do nonmetals Lose or Gain electrons? (Lose) (Gain)

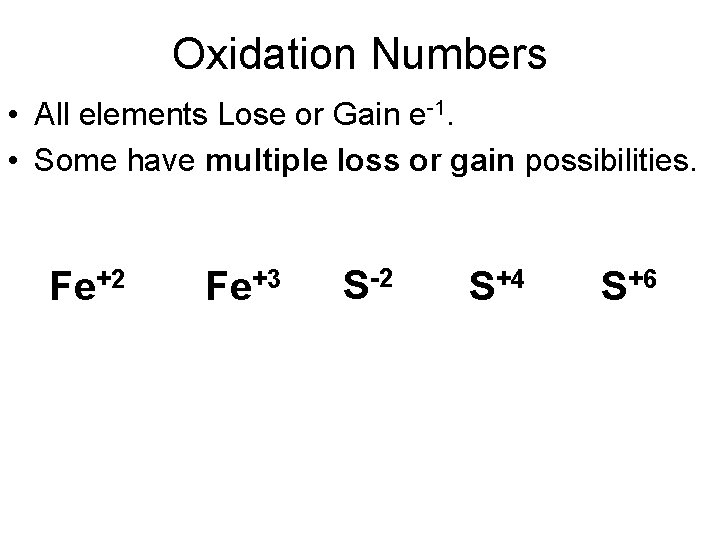
Oxidation Numbers • All elements Lose or Gain e-1. • Some have multiple loss or gain possibilities. Fe+2 Fe+3 S-2 S+4 S+6

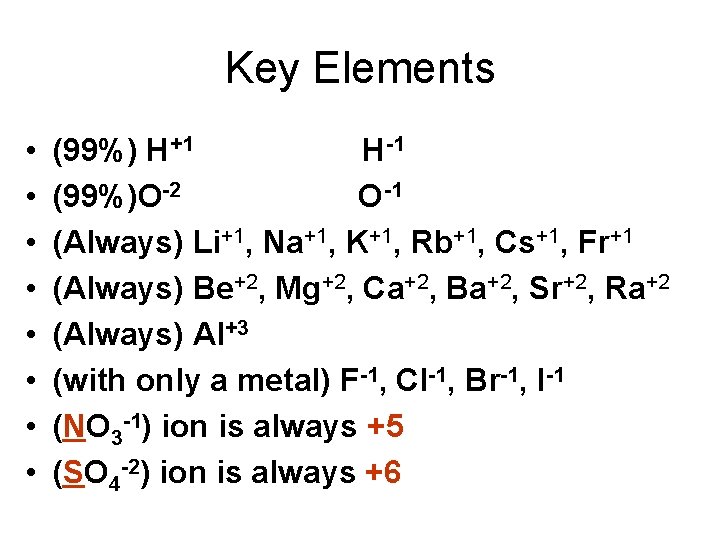
Key Elements • • (99%) H+1 H-1 (99%)O-2 O-1 (Always) Li+1, Na+1, K+1, Rb+1, Cs+1, Fr+1 (Always) Be+2, Mg+2, Ca+2, Ba+2, Sr+2, Ra+2 (Always) Al+3 (with only a metal) F-1, Cl-1, Br-1, I-1 (NO 3 -1) ion is always +5 (SO 4 -2) ion is always +6

Example One • Find the oxidation numbers. • Al 2 S 3 • Algebra is useful! 2(Al) + 3(S)= 0 • Al+3 key element • 2(+3) + 3(S)=0 • S= -2

Example Two • Find the oxidation numbers. • Ca(NO 3)2 • Algebra is useful ! (Ca)+ 2(N)+ 6(O)= 0 • Ca+2 and O-2 key elements • (+2)+2(N)+6(-2)=0 • (+2)+2(N)+(-12)=0 • 2(N)+(-10)=0 • 2(N)=10 • N= +5

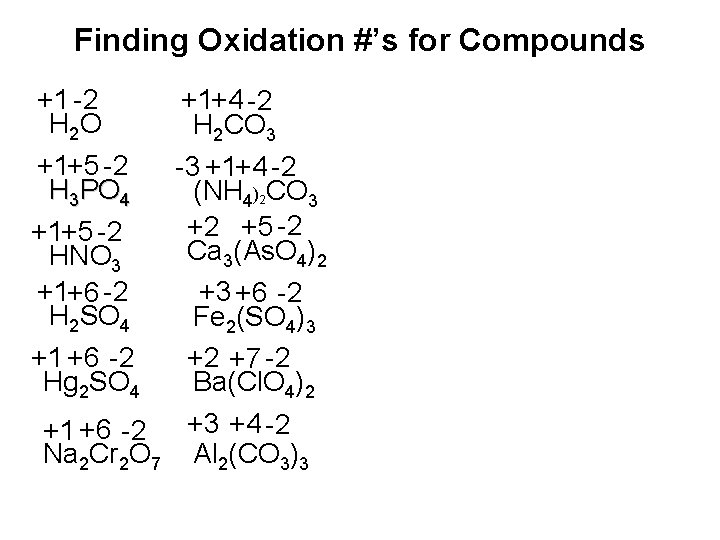
Finding Oxidation #’s for Compounds +1 -2 H 2 O +1+5 -2 H 3 PO 4 +1+5 -2 HNO 3 +1+6 -2 H 2 SO 4 +1 +6 -2 Hg 2 SO 4 +1+4 -2 H 2 CO 3 -3 +1+4 -2 (NH 4)2 CO 3 +2 +5 -2 Ca 3(As. O 4)2 +3 +6 -2 Fe 2(SO 4)3 +2 +7 -2 Ba(Cl. O 4)2 +1 +6 -2 +3 +4 -2 Na 2 Cr 2 O 7 Al 2(CO 3)3

Now it is time for class work !!! • A 101: paper practice (Work Together) • It will be due at beginning of class next time • Ready Set Break !!!

So Let’s Review • When we are assigning oxidation numbers: If we are dealing with a molecule and there is no charge, the sum of all the oxidation numbers will add to zero. If we are dealing with a Polyatomic ion, then the sum of all of the oxidation numbers adds up to that charge +2 +5 -2 +6 -2 Ca 3(PO 4)2 Cr 2 O 7 -2

CA 101 Assigning oxidation #’s Turn on Mac: double click on the MAC folder on the desktop. Open the Redox folder and click on the yellow, red and blue icon. Choose the top button: finding oxidation Numbers in triatomic compounds.

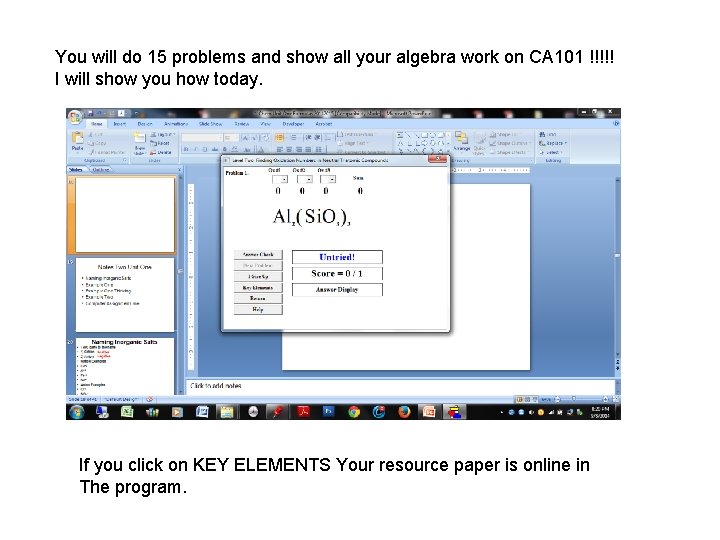
You will do 15 problems and show all your algebra work on CA 101 !!!!! I will show you how today. If you click on KEY ELEMENTS Your resource paper is online in The program.

Please get A 101 and CA 101 Stamped Today !!! Once you are done we will intro Formula Writing

Notes Two Unit One • • • Naming Inorganic Salts Example One Thinking Example Two Computer Assignment One

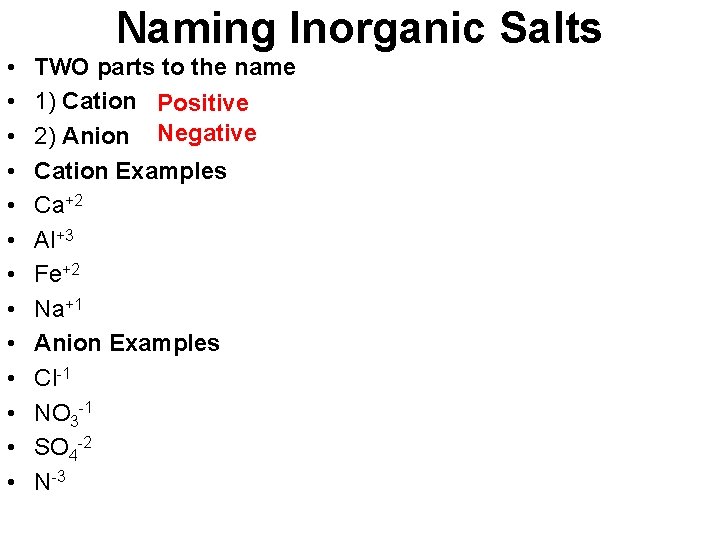
Naming Inorganic Salts • • • • TWO parts to the name 1) Cation Positive 2) Anion Negative Cation Examples Ca+2 Al+3 Fe+2 Na+1 Anion Examples Cl-1 NO 3 -1 SO 4 -2 N-3

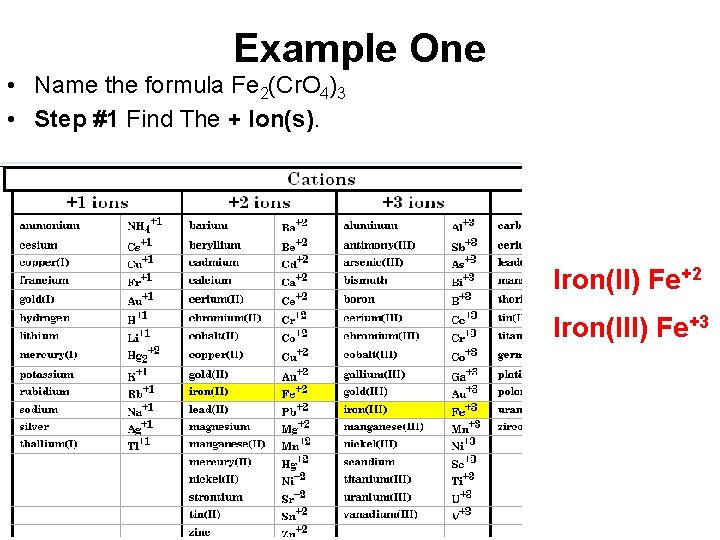
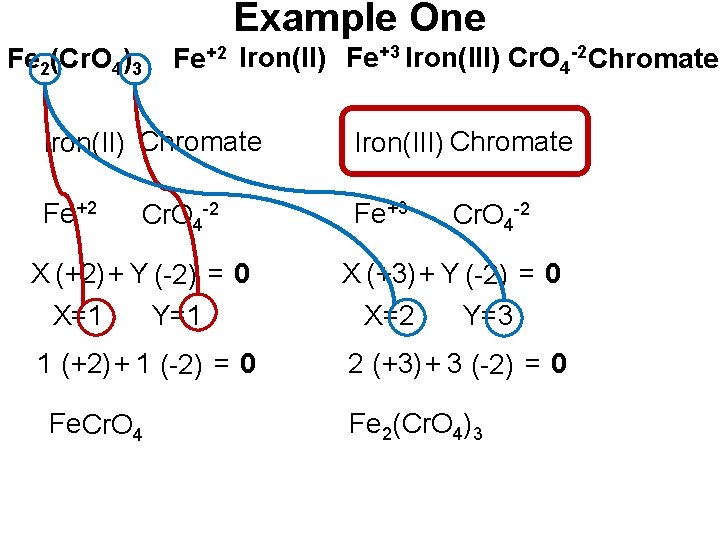
Example One • Name the formula Fe 2(Cr. O 4)3 • Step #1 Find The + Ion(s). Iron(II) Fe+2 Iron(III) Fe+3

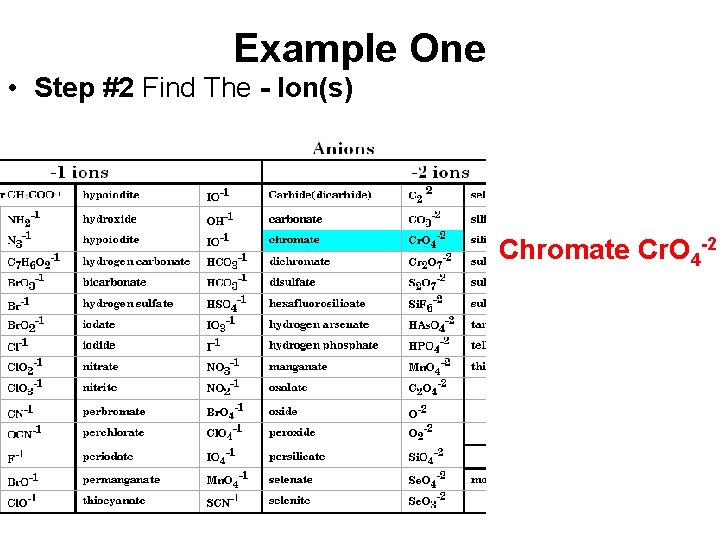
Example One • Step #2 Find The - Ion(s) Chromate Cr. O 4 -2

Example One Fe 2(Cr. O 4)3 Fe+2 Iron(II) Fe+3 Iron(III) Cr. O 4 -2 Chromate Iron(II) Chromate Iron(III) Chromate Fe+2 Fe+3 Cr. O 4 -2 X (+2) + Y (-2) = 0 X=1 Y=1 1 (+2) + 1 (-2) = 0 Fe. Cr. O 4 -2 X (+3) + Y (-2) = 0 X=2 Y=3 2 (+3) + 3 (-2) = 0 Fe 2(Cr. O 4)3

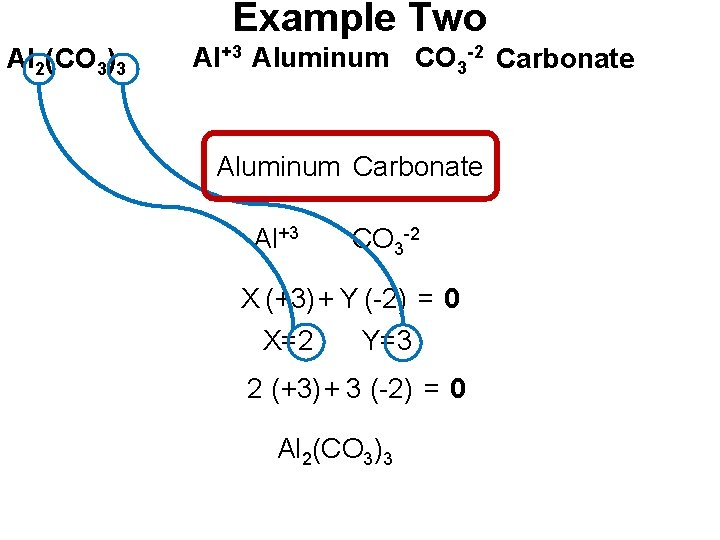
Example Two Al 2(CO 3)3 Al+3 Aluminum CO 3 -2 Carbonate Aluminum Carbonate Al+3 CO 3 -2 X (+3) + Y (-2) = 0 X=2 Y=3 2 (+3) + 3 (-2) = 0 Al 2(CO 3)3

In class work today: • Work on A 102 • Use you Cation/Anion Sheet • Remember to fnd the charges of the ions and make sure that they add up to zero to write the formula. • When you are naming a formula you always write the + ion first then the -ion

CA 102: Writing Formula CA 103: Naming Compounds • NAMING IONIC COMPOUNDS LEVELs ONE AND TWO • Get A 102 , CA 102, CA 103 all stamped today. • Now let’s look at some examples at how to write Formulas from a name. You need to have your Cation/Anion sheet out. • Homework for tonight, read L 101 Electrolysis of water Lab, we will do that next class !!!!

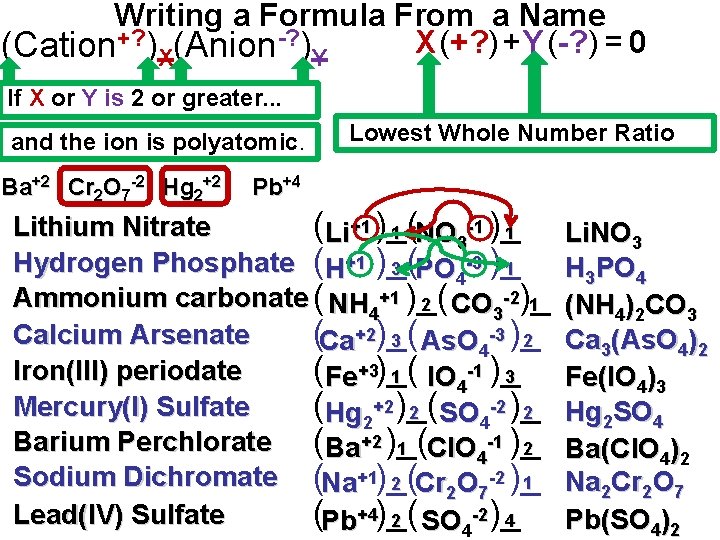
Writing a Formula From a Name X (+? ) + Y (-? ) = 0 (Cation+? )X(Anion-? )Y If X or Y is 2 or greater. . . and the ion is polyatomic. Ba+2 Cr 2 O 7 -2 Hg 2+2 Lowest Whole Number Ratio Pb+4 Lithium Nitrate (Li+1 )_( 1 NO -1 )_ 1 3 Hydrogen Phosphate (H+1 )_( 3 PO -3 )_ 1 4 Ammonium carbonate ( NH 4+1 )_( 1 2 CO 3 -2)_ Calcium Arsenate (Ca+2)_( 2 3 As. O -3 )_ 4 Iron(III) periodate (Fe+3)_( 1 IO -1 )_ 3 4 Mercury(I) Sulfate (Hg 2+2 )_( 2 SO -2 )_ 2 4 Barium Perchlorate (Ba+2 )_( 2 1 Cl. O 4 -1 )_ Sodium Dichromate (Na+1)_( 1 2 Cr O -2 )_ 2 7 Lead(IV) Sulfate (Pb+4)_( 4 2 SO -2)_ 4 Li. NO 3 H 3 PO 4 (NH 4)2 CO 3 Ca 3(As. O 4)2 Fe(IO 4)3 Hg 2 SO 4 Ba(Cl. O 4)2 Na 2 Cr 2 O 7 Pb(SO 4)2

Notes Three Unit One • • • Standard Amounts One Gopher One Mole Formula mass Percent Composition Empirical Formula

Standard Amounts How many dollars is… A) 120 pennies? 1. 2 dollars B) 2 quarters? 0. 5 dollars C) 15 nickels? 0. 75 dollars How many dozens is… D) 48 eggs? 4 dozen E)18 apple fritters 1. 5 dozen

One Dozen You KNOW it equals 12 items What if I asked you to Go into the lab and get 12 Carbon atoms?

One Mole One mole is 6. 022 x 10+23 items. Each element on the period table has a mass per mole. N 14. 0 g 6. 022 x 10+23 atoms O 16. 0 g 6. 022 x 10+23 atoms C 12. 0 g 6. 022 x 10+23 atoms How many moles are in each? How many atoms are in each? N 7. 0 g ÷ 14. 0 g/m =0. 50 m x 6. 022 x 10+23 atoms/m =3. 01 x 10+23 atoms O 4. 0 g ÷ 16. 0 g/m =0. 25 m x 6. 022 x 10+23 atoms/m =1. 51 x 10+23 atoms C 18. 0 g÷ 12. 0 g/m=1. 50 m x 6. 022 x 10+23 atoms/m =9. 03 x 10+23 atoms

Calculations Bases on Chemical Formulas • Formula mass (Molecular Mass or Gram-Formula Mass) • Empirical Formula • Percent Composition

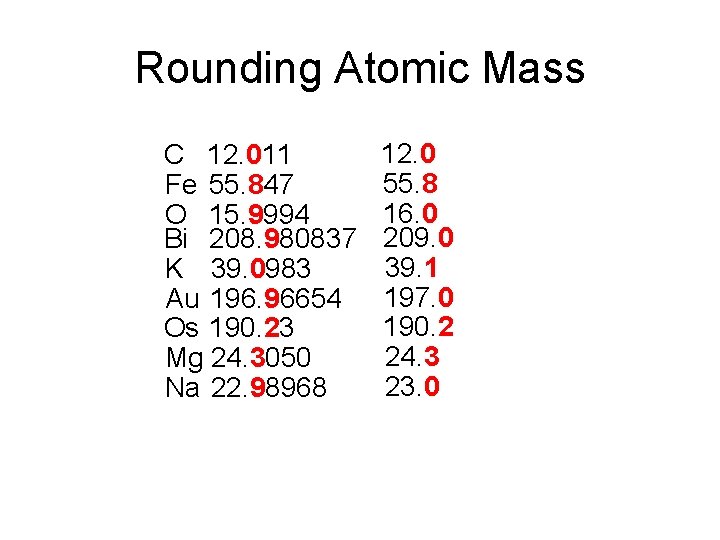
Rounding Atomic Mass C 12. 011 Fe 55. 847 O 15. 9994 Bi 208. 980837 K 39. 0983 Au 196. 96654 Os 190. 23 Mg 24. 3050 Na 22. 98968 12. 0 55. 8 16. 0 209. 0 39. 1 197. 0 190. 2 24. 3 23. 0

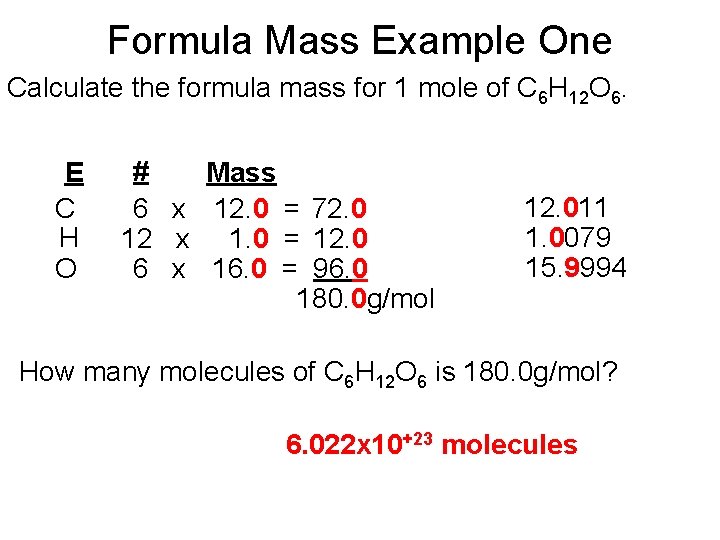
Formula Mass Example One Calculate the formula mass for 1 mole of C 6 H 12 O 6. E C H O # Mass 6 x 12. 0 = 72. 0 12 x 1. 0 = 12. 0 6 x 16. 0 = 96. 0 180. 0 g/mol 12. 011 1. 0079 15. 9994 How many molecules of C 6 H 12 O 6 is 180. 0 g/mol? 6. 022 x 10+23 molecules

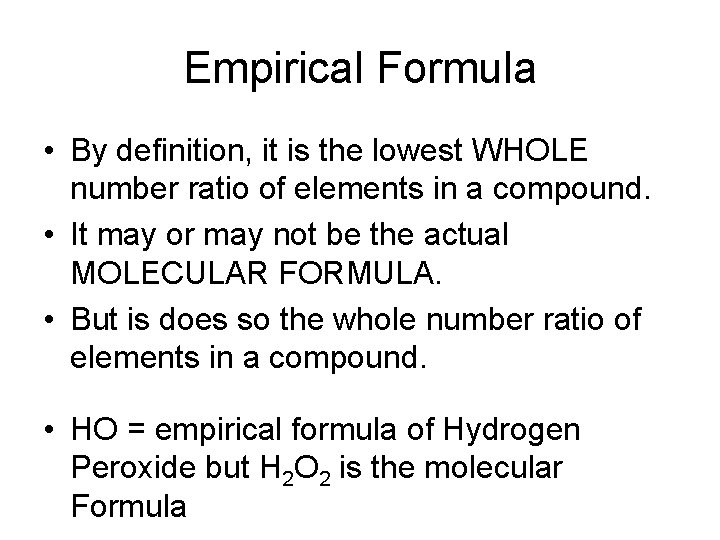
Empirical Formula • By definition, it is the lowest WHOLE number ratio of elements in a compound. • It may or may not be the actual MOLECULAR FORMULA. • But is does so the whole number ratio of elements in a compound. • HO = empirical formula of Hydrogen Peroxide but H 2 O 2 is the molecular Formula

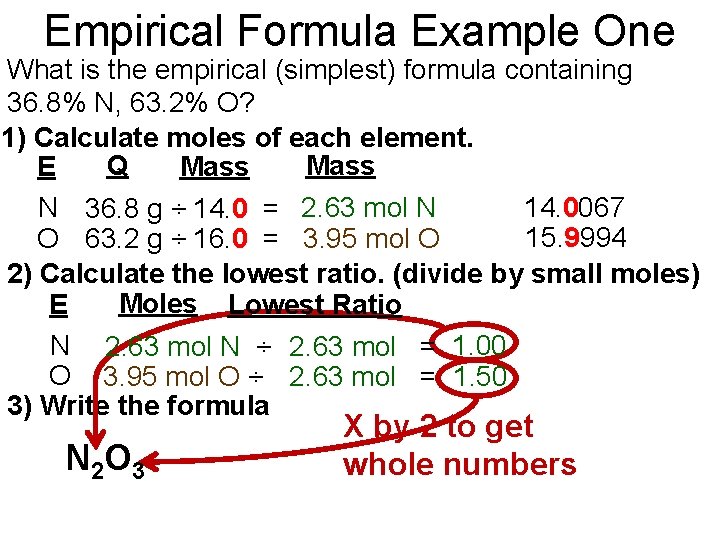
Empirical Formula Example One What is the empirical (simplest) formula containing 36. 8% N, 63. 2% O? 1) Calculate moles of each element. Q Mass E Mass N 36. 8 g ÷ 14. 0 = 2. 63 mol N 14. 0067 15. 9994 O 63. 2 g ÷ 16. 0 = 3. 95 mol O 2) Calculate the lowest ratio. (divide by small moles) Moles Lowest Ratio E N 2. 63 mol N ÷ 2. 63 mol = 1. 00 O 3. 95 mol O ÷ 2. 63 mol = 1. 50 3) Write the formula N 2 O 3 X by 2 to get whole numbers

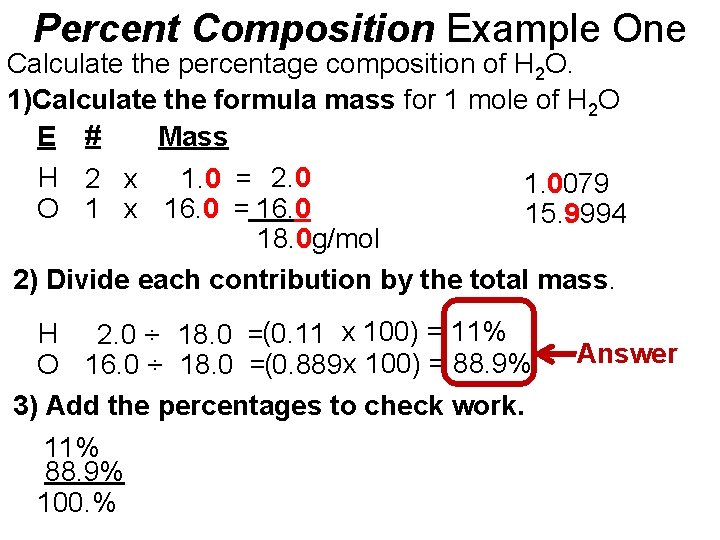
Percent Composition Example One Calculate the percentage composition of H 2 O. 1)Calculate the formula mass for 1 mole of H 2 O E # Mass H 2 x 1. 0 = 2. 0 1. 0079 O 1 x 16. 0 = 16. 0 15. 9994 18. 0 g/mol 2) Divide each contribution by the total mass. H 2. 0 ÷ 18. 0 =(0. 11 x 100) = 11% O 16. 0 ÷ 18. 0 =(0. 889 x 100) = 88. 9% 3) Add the percentages to check work. 11% 88. 9% 100. % Answer

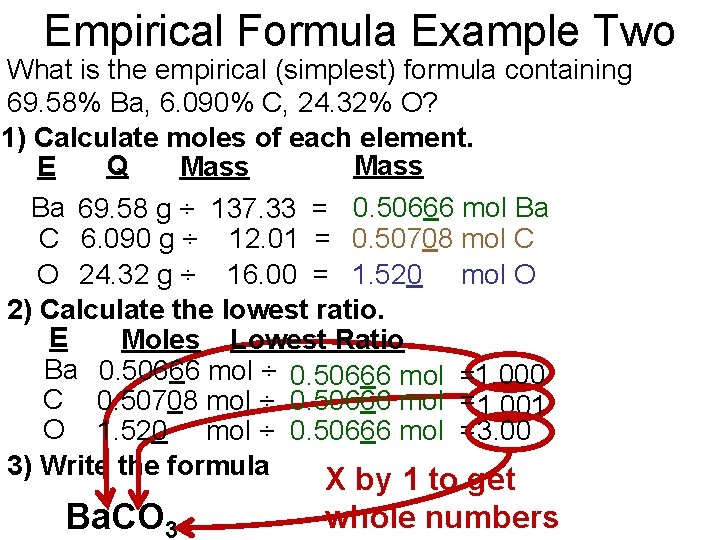
Empirical Formula Example Two What is the empirical (simplest) formula containing 69. 58% Ba, 6. 090% C, 24. 32% O? 1) Calculate moles of each element. Q Mass E Mass Ba 69. 58 g ÷ 137. 33 = 0. 50666 mol Ba C 6. 090 g ÷ 12. 01 = 0. 50708 mol C O 24. 32 g ÷ 16. 00 = 1. 520 mol O 2) Calculate the lowest ratio. E Moles Lowest Ratio Ba 0. 50666 mol ÷ 0. 50666 mol =1. 000 C 0. 50708 mol ÷ 0. 50666 mol =1. 001 O 1. 520 mol ÷ 0. 50666 mol =3. 00 3) Write the formula Ba. CO 3 X by 1 to get whole numbers

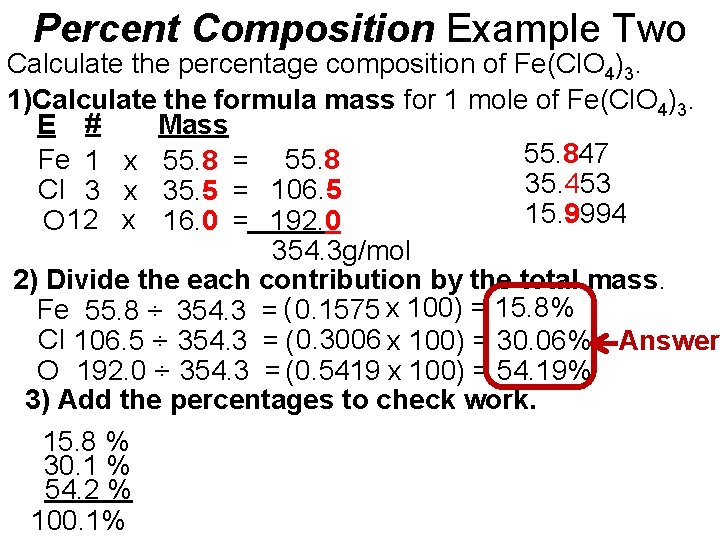
Percent Composition Example Two Calculate the percentage composition of Fe(Cl. O 4)3. 1)Calculate the formula mass for 1 mole of Fe(Cl. O 4)3. E # Mass 55. 847 Fe 1 x 55. 8 = 55. 8 35. 453 Cl 3 x 35. 5 = 106. 5 15. 9994 O 12 x 16. 0 = 192. 0 354. 3 g/mol 2) Divide the each contribution by the total mass. Fe 55. 8 ÷ 354. 3 = ( 0. 1575 x 100) = 15. 8% Cl 106. 5 ÷ 354. 3 = ( 0. 3006 x 100) = 30. 06% Answer O 192. 0 ÷ 354. 3 = (0. 5419 x 100) = 54. 19% 3) Add the percentages to check work. 15. 8 % 30. 1 % 54. 2 % 100. 1%

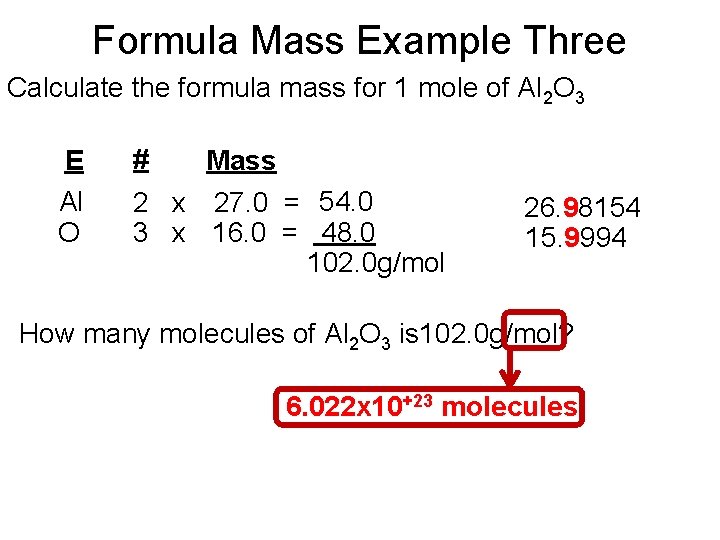
Formula Mass Example Three Calculate the formula mass for 1 mole of Al 2 O 3 E Al O # Mass 2 x 27. 0 = 54. 0 3 x 16. 0 = 48. 0 102. 0 g/mol 26. 98154 15. 9994 How many molecules of Al 2 O 3 is 102. 0 g/mol? 6. 022 x 10+23 molecules

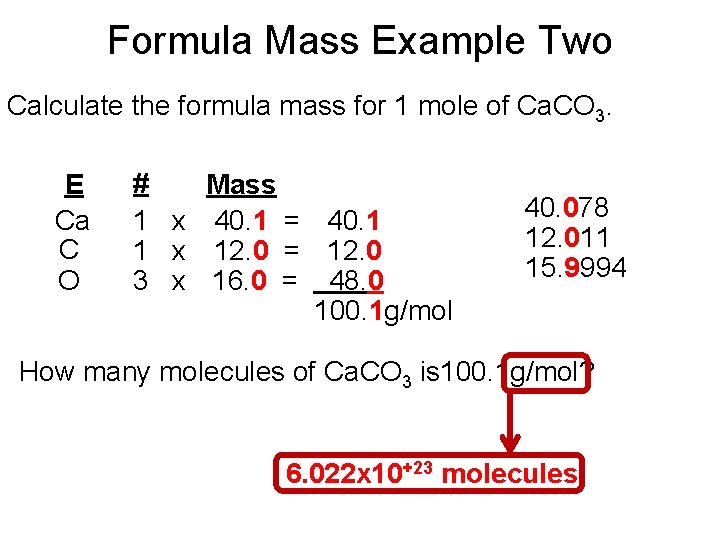
Formula Mass Example Two Calculate the formula mass for 1 mole of Ca. CO 3. E Ca C O # Mass 1 x 40. 1 = 40. 1 1 x 12. 0 = 12. 0 3 x 16. 0 = 48. 0 100. 1 g/mol 40. 078 12. 011 15. 9994 How many molecules of Ca. CO 3 is 100. 1 g/mol? 6. 022 x 10+23 molecules