Unit IV Chemical Equilibrium Focusing on AcidBase Systems
Unit IV Chemical Equilibrium Focusing on Acid-Base Systems
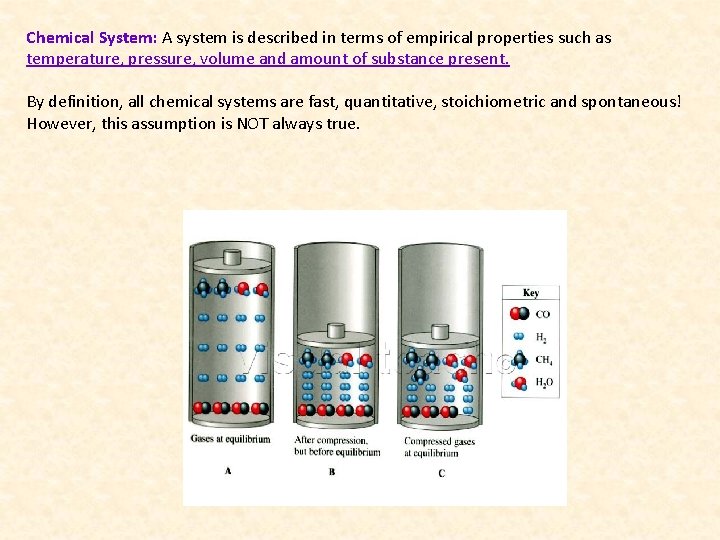
Chemical System: A system is described in terms of empirical properties such as temperature, pressure, volume and amount of substance present. By definition, all chemical systems are fast, quantitative, stoichiometric and spontaneous! However, this assumption is NOT always true.

A chemical system that is separate from it’s surroundings where no matter can enter or leave is called a closed system. When a bottle of carbonated beverage is opened, the pressure on the system changes and dissolved gas is allowed to leave the system. The equilibrium has been disturbed.
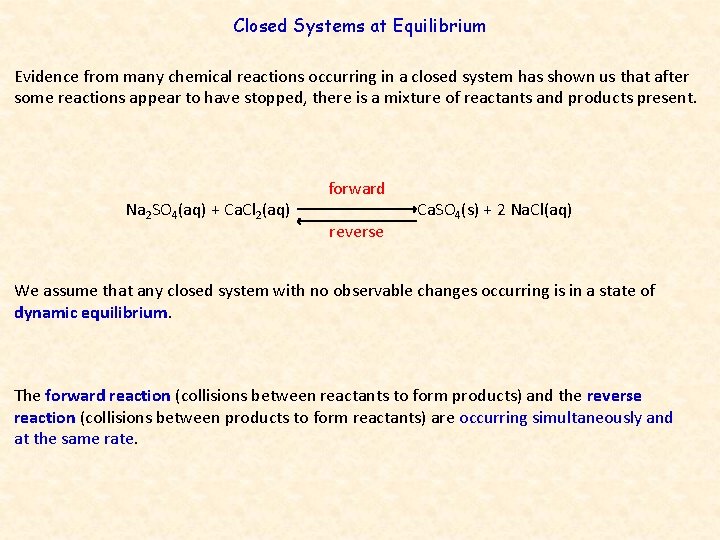
Closed Systems at Equilibrium Evidence from many chemical reactions occurring in a closed system has shown us that after some reactions appear to have stopped, there is a mixture of reactants and products present. Na 2 SO 4(aq) + Ca. Cl 2(aq) forward reverse Ca. SO 4(s) + 2 Na. Cl(aq) We assume that any closed system with no observable changes occurring is in a state of dynamic equilibrium. The forward reaction (collisions between reactants to form products) and the reverse reaction (collisions between products to form reactants) are occurring simultaneously and at the same rate.
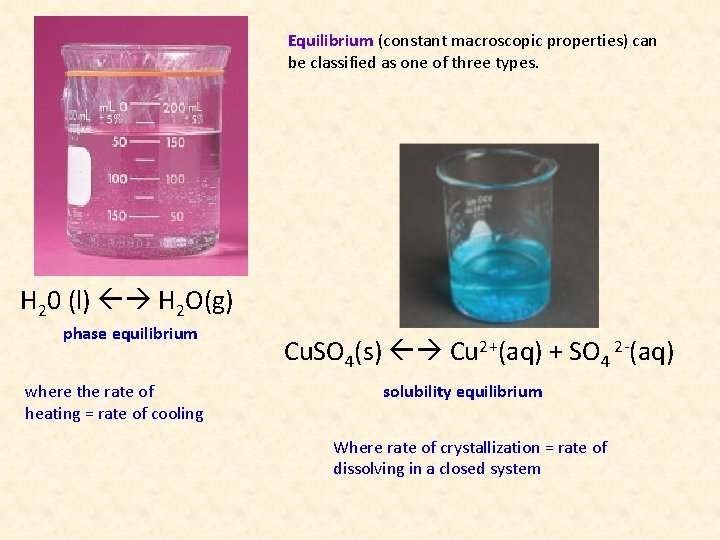
Equilibrium (constant macroscopic properties) can be classified as one of three types. H 20 (l) H 2 O(g) phase equilibrium where the rate of heating = rate of cooling Cu. SO 4(s) Cu 2+(aq) + SO 4 2 -(aq) solubility equilibrium Where rate of crystallization = rate of dissolving in a closed system
Chemical Reaction Equilibrium H 2(g) + I 2(g) 2 HI(g), t = 448°C
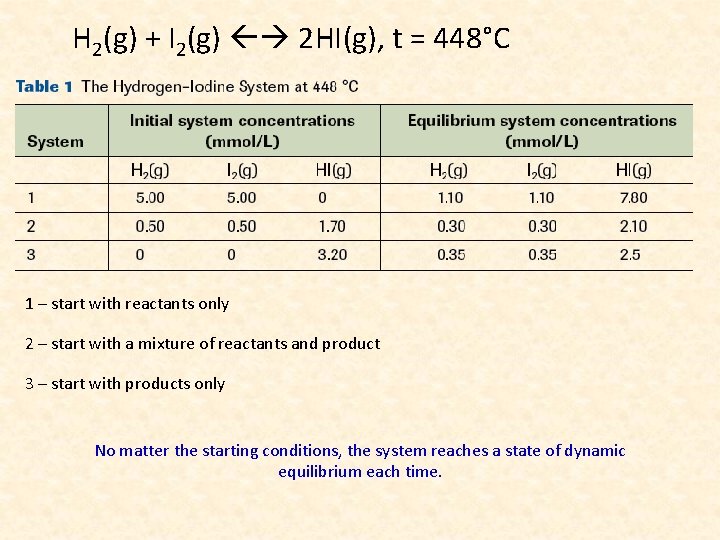
H 2(g) + I 2(g) 2 HI(g), t = 448°C 1 – start with reactants only 2 – start with a mixture of reactants and product 3 – start with products only No matter the starting conditions, the system reaches a state of dynamic equilibrium each time.
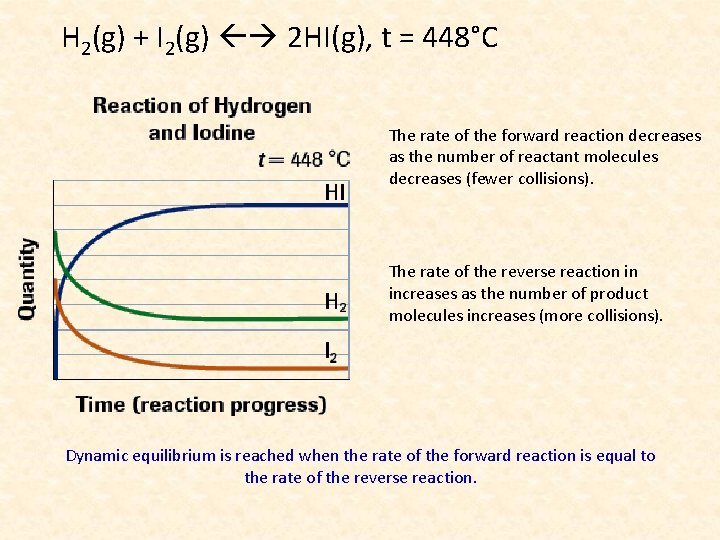
H 2(g) + I 2(g) 2 HI(g), t = 448°C The rate of the forward reaction decreases as the number of reactant molecules decreases (fewer collisions). The rate of the reverse reaction in increases as the number of product molecules increases (more collisions). Dynamic equilibrium is reached when the rate of the forward reaction is equal to the rate of the reverse reaction.
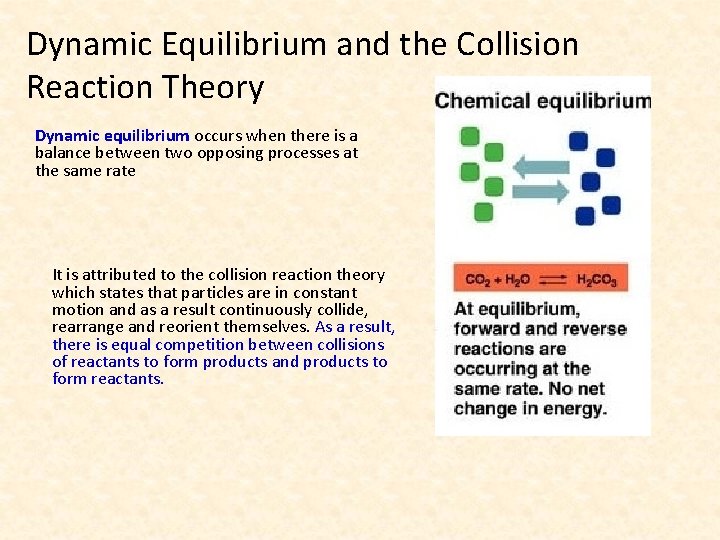
Dynamic Equilibrium and the Collision Reaction Theory Dynamic equilibrium occurs when there is a balance between two opposing processes at the same rate It is attributed to the collision reaction theory which states that particles are in constant motion and as a result continuously collide, rearrange and reorient themselves. As a result, there is equal competition between collisions of reactants to form products and products to form reactants.
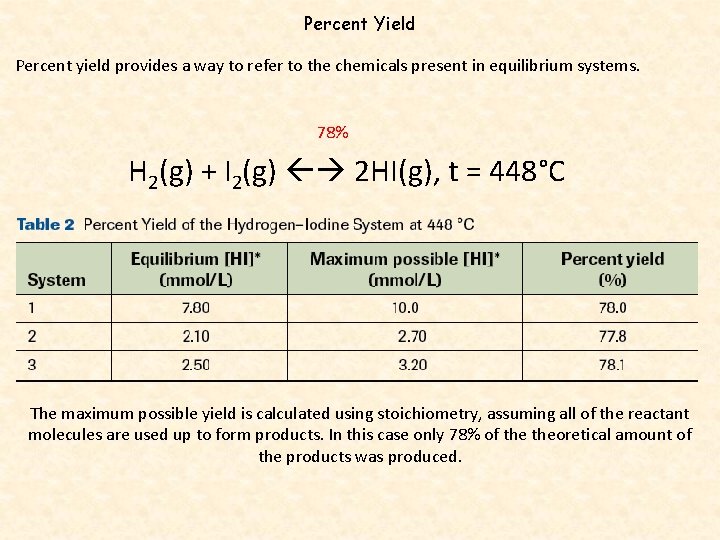
Percent Yield Percent yield provides a way to refer to the chemicals present in equilibrium systems. 78% H 2(g) + I 2(g) 2 HI(g), t = 448°C The maximum possible yield is calculated using stoichiometry, assuming all of the reactant molecules are used up to form products. In this case only 78% of theoretical amount of the products was produced.
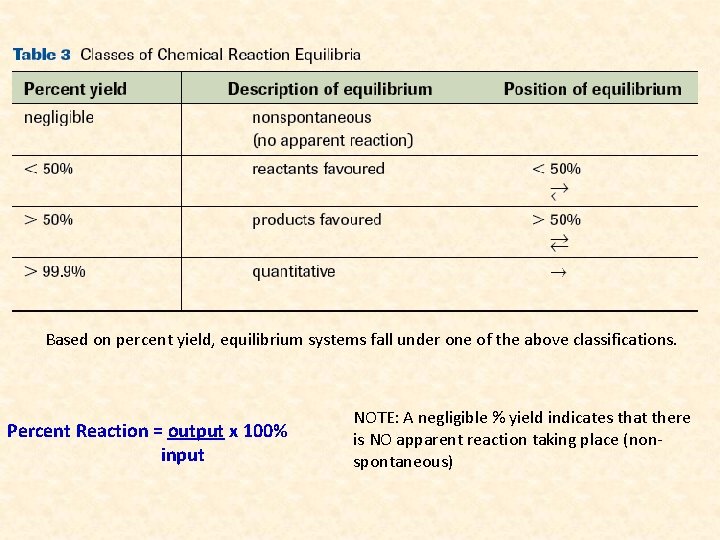
Based on percent yield, equilibrium systems fall under one of the above classifications. Percent Reaction = output x 100% input NOTE: A negligible % yield indicates that there is NO apparent reaction taking place (nonspontaneous)
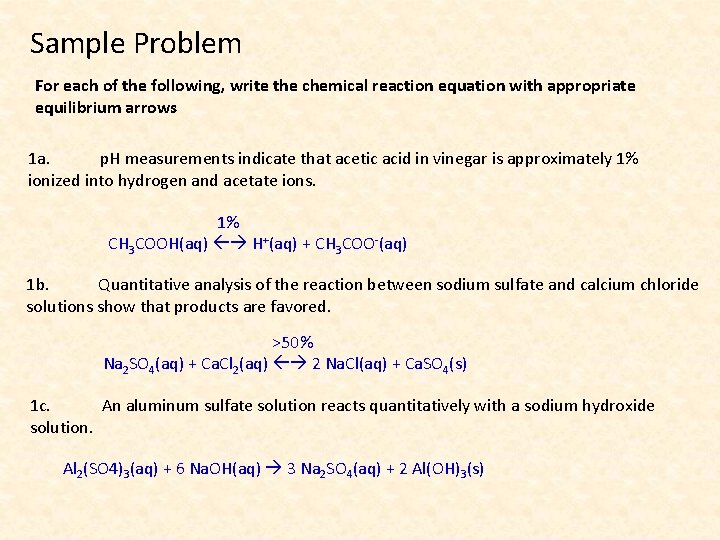
Sample Problem For each of the following, write the chemical reaction equation with appropriate equilibrium arrows 1 a. p. H measurements indicate that acetic acid in vinegar is approximately 1% ionized into hydrogen and acetate ions. 1% CH 3 COOH(aq) H+(aq) + CH 3 COO-(aq) 1 b. Quantitative analysis of the reaction between sodium sulfate and calcium chloride solutions show that products are favored. >50% Na 2 SO 4(aq) + Ca. Cl 2(aq) 2 Na. Cl(aq) + Ca. SO 4(s) 1 c. An aluminum sulfate solution reacts quantitatively with a sodium hydroxide solution. Al 2(SO 4)3(aq) + 6 Na. OH(aq) 3 Na 2 SO 4(aq) + 2 Al(OH)3(s)

Sample Problem When 1. 5 mol of chlorine gas was mixed with excess carbon monoxide in a 1. 00 L container, 0. 80 mol of COCl 2(g) was produced at equilibrium. Determine the % reaction.
Equilibrium Law and the Equilibrium Constant (Kc) According to equilibrium law, when a chemical system is at equilibrium, the value for this equilibrium is constant. An equilibrium law expression is based on a balanced equation for the reaction system. Balance the equation using whole number coefficients and IGNORE the concentrations of PURE solids and liquids!!! The equilibrium constant is a unitless numerical value that is mathematically equal to the concentration of all product species, divided by the concentration of all reactant species, raised to the power of the co-efficient of that species
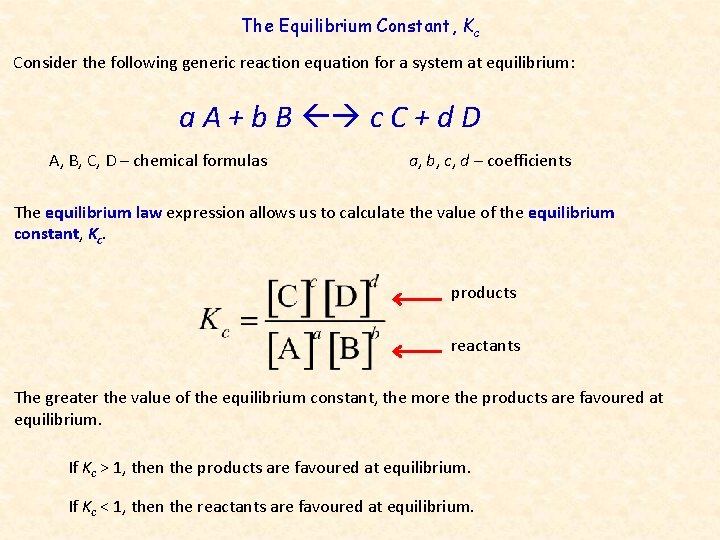
The Equilibrium Constant, Kc Consider the following generic reaction equation for a system at equilibrium: a A + b B c C + d D A, B, C, D – chemical formulas a, b, c, d – coefficients The equilibrium law expression allows us to calculate the value of the equilibrium constant, Kc. products reactants The greater the value of the equilibrium constant, the more the products are favoured at equilibrium. If Kc > 1, then the products are favoured at equilibrium. If Kc < 1, then the reactants are favoured at equilibrium.

Guidelines for Determining Kc Pure SOLIDS are NOT included in the equilibrium constant formula since the concentration (density) does not vary. 2 Na. HCO 3(s) Na 2 CO 3(s) + H 2 O(g) + CO 2(g)

Guidelines for Determining Kc Pure LIQUIDS are NOT included in the equilibrium constant since the concentration (density) does not vary. A pure liquid occurs when two different elements combine to produce a liquid; otherwise the liquid is classified as a homogeneous mixture. 2 H 2(g) + O 2(g) 2 H 2 O(l)

Guidelines for Determining Kc Aqueous IONS and GASES in solution ARE included since the concentration of aqueous ions and gases can be altered by varying the volume of solvent. N 2 H 4(g) + H 2 O(l) N 2 H 5+(aq) + OH-(aq)
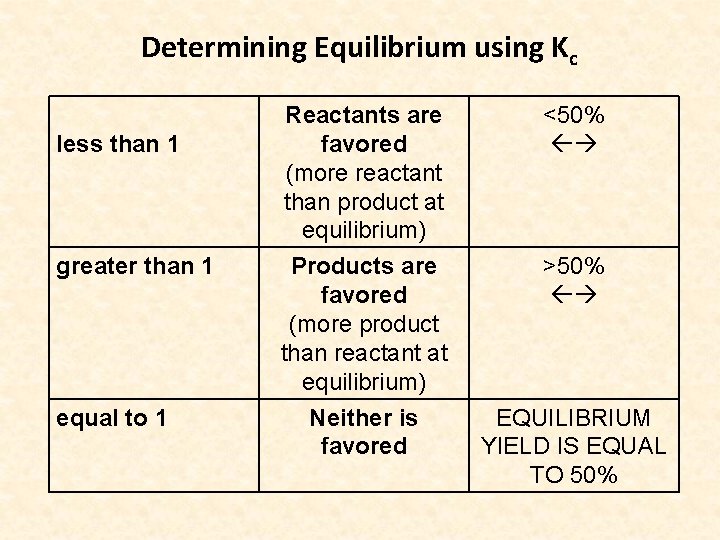
Determining Equilibrium using Kc less than 1 greater than 1 equal to 1 Reactants are favored (more reactant than product at equilibrium) <50% Products are favored (more product than reactant at equilibrium) Neither is favored >50% EQUILIBRIUM YIELD IS EQUAL TO 50%

Note: An equilibrium constant value is dependent upon: • The systems temperature An equilibrium constant value is independent upon: • The reagent concentration • Any catalyst present • The time taken to reach equilibrium
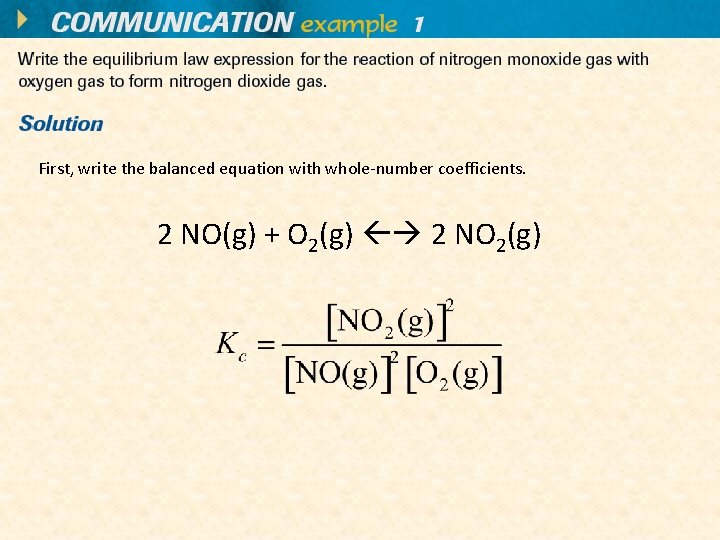
First, write the balanced equation with whole-number coefficients. 2 NO(g) + O 2(g) 2 NO 2(g)
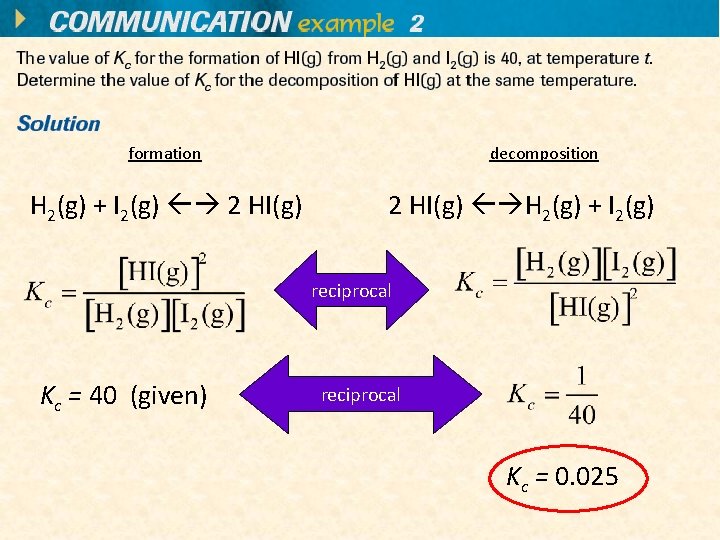
formation H 2(g) + I 2(g) 2 HI(g) decomposition 2 HI(g) H 2(g) + I 2(g) reciprocal Kc = 40 (given) reciprocal Kc = 0. 025
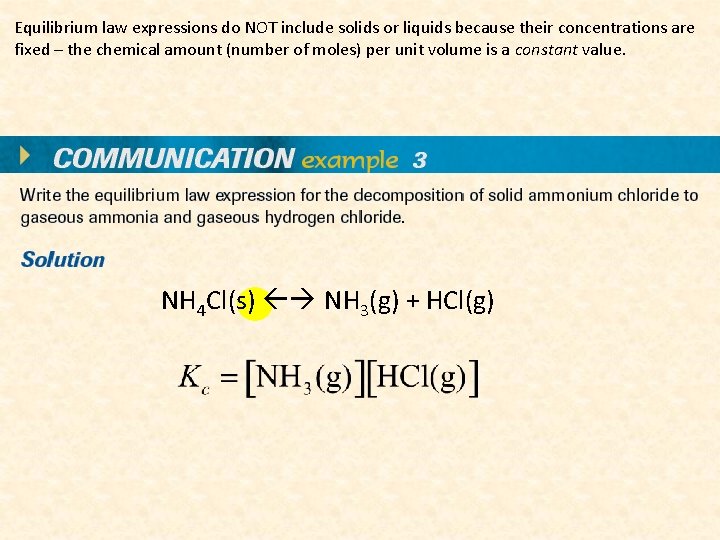
Equilibrium law expressions do NOT include solids or liquids because their concentrations are fixed – the chemical amount (number of moles) per unit volume is a constant value. NH 4 Cl(s) NH 3(g) + HCl(g)
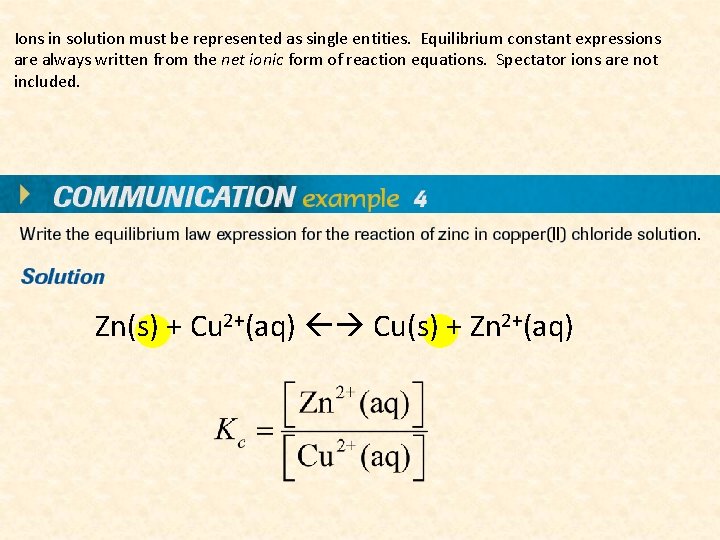
Ions in solution must be represented as single entities. Equilibrium constant expressions are always written from the net ionic form of reaction equations. Spectator ions are not included. Zn(s) + Cu 2+(aq) Cu(s) + Zn 2+(aq)
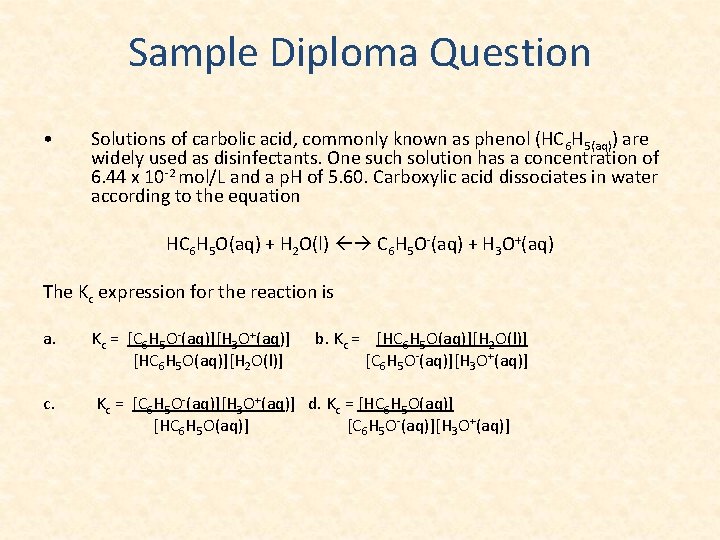
Sample Diploma Question • Solutions of carbolic acid, commonly known as phenol (HC 6 H 5(aq)) are widely used as disinfectants. One such solution has a concentration of 6. 44 x 10 -2 mol/L and a p. H of 5. 60. Carboxylic acid dissociates in water according to the equation HC 6 H 5 O(aq) + H 2 O(l) C 6 H 5 O-(aq) + H 3 O+(aq) The Kc expression for the reaction is a. Kc = [C 6 H 5 O-(aq)][H 3 O+(aq)] [HC 6 H 5 O(aq)][H 2 O(l)] c. Kc = [C 6 H 5 O-(aq)][H 3 O+(aq)] d. Kc = [HC 6 H 5 O(aq)] [C 6 H 5 O-(aq)][H 3 O+(aq)] b. Kc = [HC 6 H 5 O(aq)][H 2 O(l)] [C 6 H 5 O-(aq)][H 3 O+(aq)]
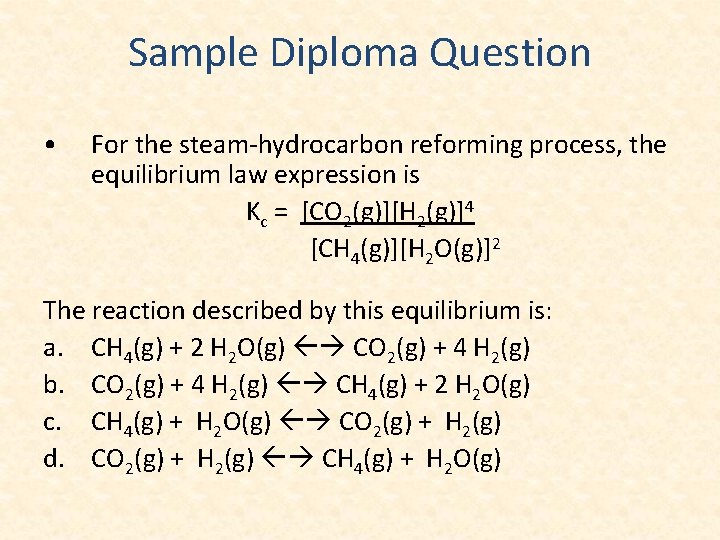
Sample Diploma Question • For the steam-hydrocarbon reforming process, the equilibrium law expression is Kc = [CO 2(g)][H 2(g)]4 [CH 4(g)][H 2 O(g)]2 The reaction described by this equilibrium is: a. CH 4(g) + 2 H 2 O(g) CO 2(g) + 4 H 2(g) b. CO 2(g) + 4 H 2(g) CH 4(g) + 2 H 2 O(g) c. CH 4(g) + H 2 O(g) CO 2(g) + H 2(g) d. CO 2(g) + H 2(g) CH 4(g) + H 2 O(g)
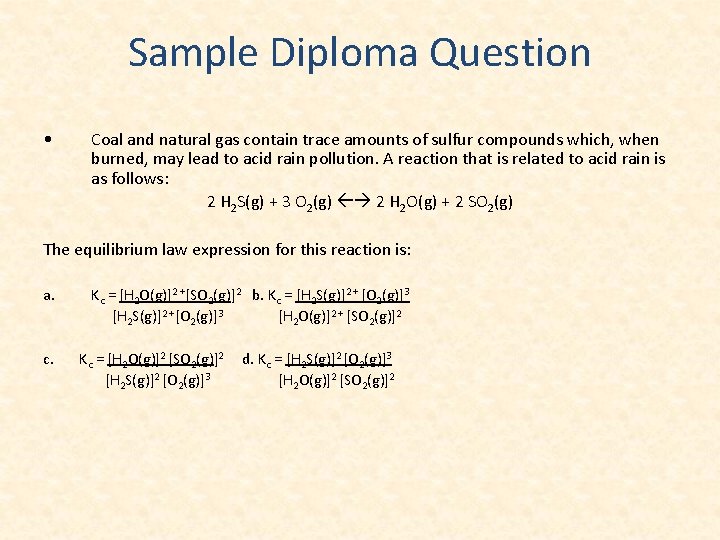
Sample Diploma Question • Coal and natural gas contain trace amounts of sulfur compounds which, when burned, may lead to acid rain pollution. A reaction that is related to acid rain is as follows: 2 H 2 S(g) + 3 O 2(g) 2 H 2 O(g) + 2 SO 2(g) The equilibrium law expression for this reaction is: a. c. Kc = [H 2 O(g)]2 +[SO 2(g)]2 b. Kc = [H 2 S(g)]2 + [O 2(g)]3 [H 2 S(g)]2 +[O 2(g)]3 [H 2 O(g)]2 + [SO 2(g)]2 Kc = [H 2 O(g)]2 [SO 2(g)]2 [H 2 S(g)]2 [O 2(g)]3 d. Kc = [H 2 S(g)]2 [O 2(g)]3 [H 2 O(g)]2 [SO 2(g)]2
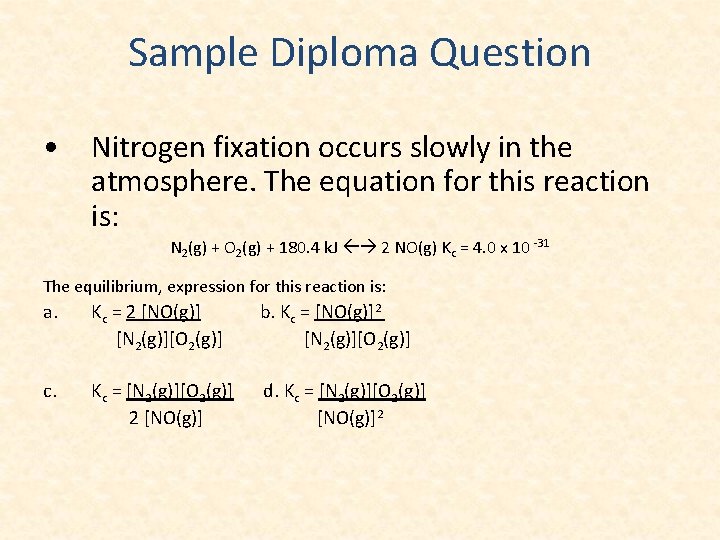
Sample Diploma Question • Nitrogen fixation occurs slowly in the atmosphere. The equation for this reaction is: N 2(g) + O 2(g) + 180. 4 k. J 2 NO(g) Kc = 4. 0 x 10 -31 The equilibrium, expression for this reaction is: a. Kc = 2 [NO(g)] [N 2(g)][O 2(g)] b. Kc = [NO(g)]2 [N 2(g)][O 2(g)] c. Kc = [N 2(g)][O 2(g)] 2 [NO(g)] d. Kc = [N 2(g)][O 2(g)] [NO(g)]2

Sample Diploma Question continued… N 2(g) + O 2(g) + 180. 4 k. J 2 NO(g) Kc = 4. 0 x 10 -31 At equilibrium, if the [O 2(g)]= [N 2(g)], then: a. [NO(g)] = [N 2(g)] b. [NO(g)] > [N 2(g)] c. [NO(g)] = 2 [N 2(g)] d. [NO(g)] < [N 2(g)]

Finding concentrations at equilibrium • In order to determine the equilibrium constant for a given reaction, equilibrium concentrations are required. If they are not given, an ICE table can be used to determine the equilibrium concentration I: Initial concentration C: Change in concentration (can be either an increase or a decrease) E: Equilibrium concentration
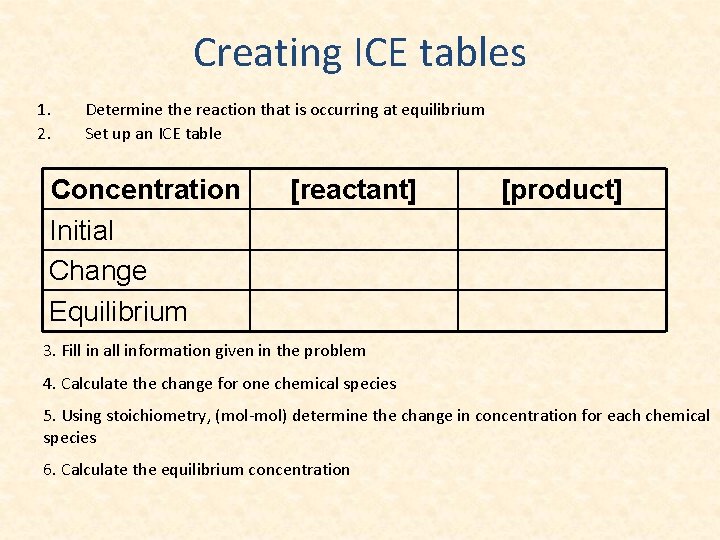
Creating ICE tables 1. 2. Determine the reaction that is occurring at equilibrium Set up an ICE table Concentration Initial Change Equilibrium [reactant] [product] 3. Fill in all information given in the problem 4. Calculate the change for one chemical species 5. Using stoichiometry, (mol-mol) determine the change in concentration for each chemical species 6. Calculate the equilibrium concentration

NOTE • Since all substances in the reaction are gases, stoichiometric calculations can involve concentration directly. This is because the volume will be the same for every gaseous substance in the container. • This is also true when every substance in a reaction is an aqueous entity dissolved in the same volume of solvent.
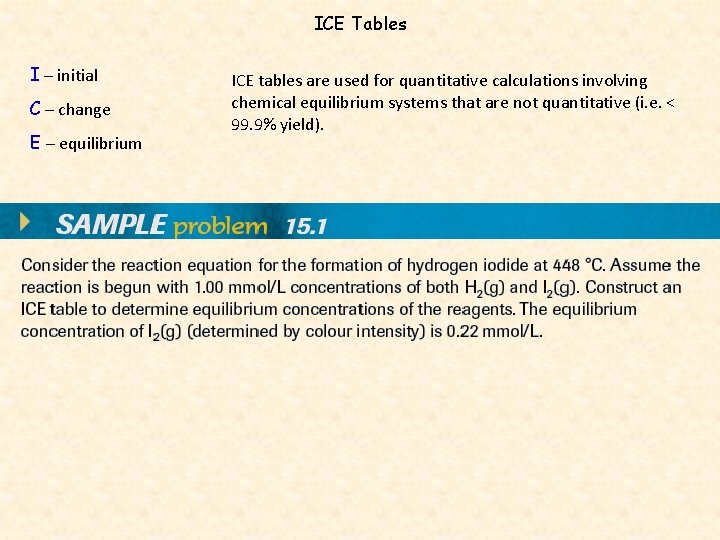
ICE Tables I – initial C – change E – equilibrium ICE tables are used for quantitative calculations involving chemical equilibrium systems that are not quantitative (i. e. < 99. 9% yield).

Predicting Final Equilibrium Concentrations
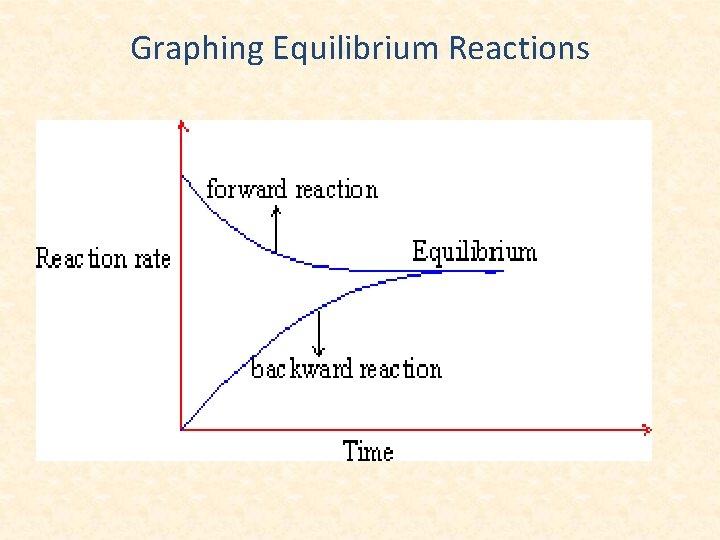
Graphing Equilibrium Reactions
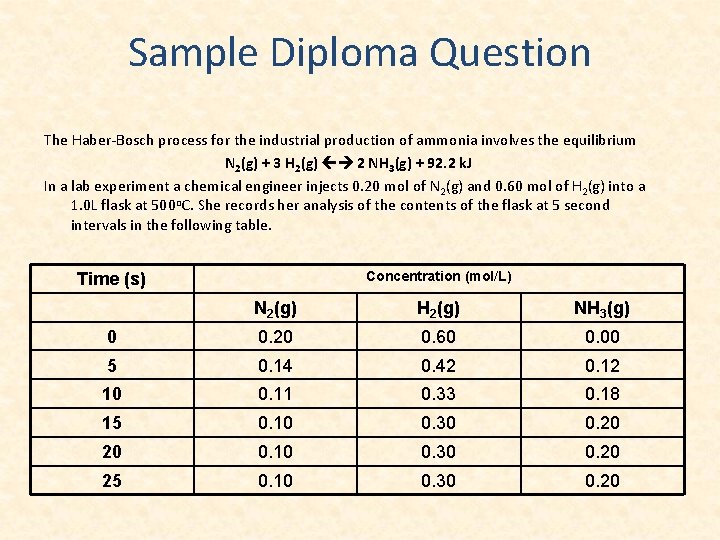
Sample Diploma Question The Haber-Bosch process for the industrial production of ammonia involves the equilibrium N 2(g) + 3 H 2(g) 2 NH 3(g) + 92. 2 k. J In a lab experiment a chemical engineer injects 0. 20 mol of N 2(g) and 0. 60 mol of H 2(g) into a 1. 0 L flask at 500 o. C. She records her analysis of the contents of the flask at 5 second intervals in the following table. Concentration (mol/L) Time (s) N 2(g) H 2(g) NH 3(g) 0 0. 20 0. 60 0. 00 5 0. 14 0. 42 0. 12 10 0. 11 0. 33 0. 18 15 0. 10 0. 30 0. 20 20 0. 10 0. 30 0. 20 25 0. 10 0. 30 0. 20
Sample diploma continued… Analyze the data. Your response should include: – A plot of the concentration of N 2(g), H 2(g) and NH 3(g), versus time on the graph paper provided – The time required to establish equilibrium – The equilibrium constant for the reaction

Sample Diploma Question

ü Read pgs. 676 – 687 ü pg. 682 Practice #’s 1 – 3 ü Lab exercise 15. A on p. 683 ü Lab exercise 15. B on p. 686 ü pgs. 688 – 689 Section 15. 1 Questions #’s 1, 3, 5 – 10
- Slides: 40