UNIT I Protein Structure and Function Globular Proteins

UNIT I: Protein Structure and Function Globular Proteins

Overview • This chapter examines relationship b/w structure and function for the clinically important globular hemeproteins, and mainly Hb.
II. Globular hemeproteins • Hemeproteins: group of specialized proteins, contain heme as a tightly bound prosthetic group • Role of heme group is dictated by environ. created by 3 D structure of protein e. g. , – Heme of a cytochrome functions as an electron carrier – Heme of catalase is part of active site of the enzyme catalyzes breakdown of H 2 O 2 – In Hb and myoglobin, the 2 most abundant heme proteins, heme serves to reversibly bind oxygen
A. Structure of heme • A complex of protoporphyrin IX and ferrous iron (Fe 2+) • Iron is held in center of heme molecule by bonds to 4 nitrogens of porphyrin ring • Heme Fe 2+ can form 2 additional bonds, one on each side of the planar porphyrin ring e. g. , in myoglobin and Hb, one of these positions is coordinated to side chain of His residue of globin molecule, the other is available to bind oxygen
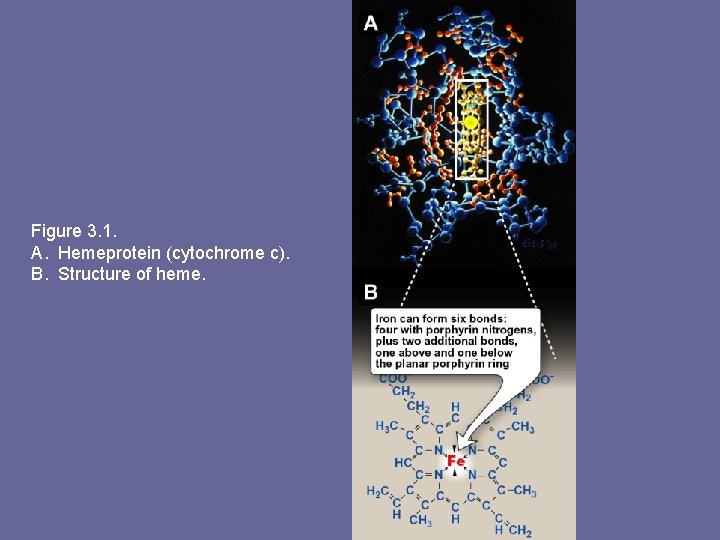
Figure 3. 1. A. Hemeprotein (cytochrome c). B. Structure of heme.
B. Structure and function of Myoglobin • Myoglobin, a hemeprotein present in heart & skeletal muscle • Functions as a reservoir for oxygen & as oxygen carrier that increases rate of transport of oxygen within muscle cell • Consists of a single polyp structurally similar to individual subunit polyp of Hb molecule, making myoglobin useful model for interpreting some complex properties of Hb

1. • • 2. • • α-helical content: Myoglobin a compact molecule, ~ 80% of its polyp folded into 8 stretches of α-helix These α-helical regions are terminated either by Pro (its 5 -membered ring cannot be accommodated in α– helix), or by β-bends and loops stabilized by H-bonds and ionic bonds Location of polar and non-polar aa residues: Interior of myoglobin molecule is composed of almost entirely non-polar aa’s. Packed together forming a structure stabilized by hydrophobic interactions Charged aa’s located almost exclusively on surface, forming H-bonds with each other and with water
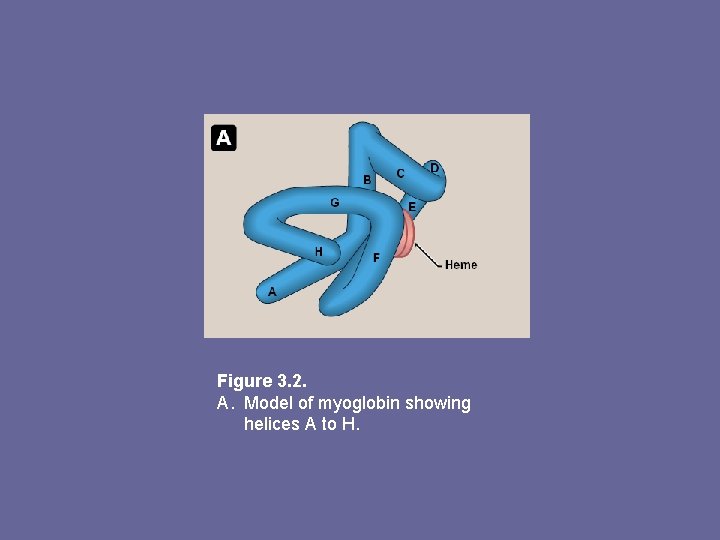
Figure 3. 2. A. Model of myoglobin showing helices A to H.

3. Binding of heme group: – – Heme group sits in a crevice lined with non-polar aa’s. Notable exceptions are 2 His residues. One, proximal His, binds directly to iron of heme, 2 nd distal His, does not directly interact with heme, but helps stabilize binding of oxygen to ferrous iron The protein, or globin, portion of myoglobin creates a microenviron. for heme that permits reversible binding of one oxygen molecule (oxygenation). Simultaneous loss of electrons by ferrous iron (oxidation) occurs only rarely.
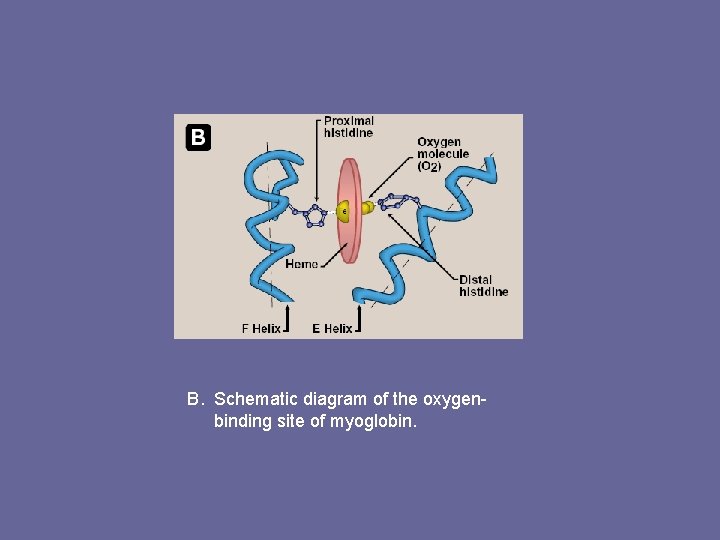
B. Schematic diagram of the oxygenbinding site of myoglobin.
C. Structure and function of hemoglobin • Hb is found exclusively in RBC’s, its main function is transport of oxygen from lungs to capillaries of tissues. Hb. A, major Hb in adults, is composed of 4 polyps. 2 αand 2 β-chains –held together by non-covalent interactions. Each subunit has stretches of α-helical structure, and a heme binding pocket. Tetrameric Hb is more complex structurally and functionally than myoglobin e. g. , • • • – – – Hb can transport CO 2 from tissues to lungs, and carry 4 O 2 from lungs to cells of the body, further Oxygen-binding properties of Hb are regulated by interaction with allosteric effectors
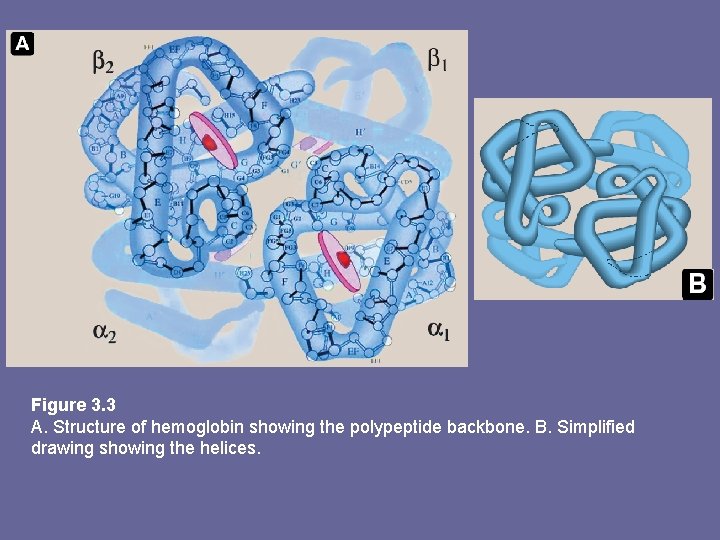
Figure 3. 3 A. Structure of hemoglobin showing the polypeptide backbone. B. Simplified drawing showing the helices.

1. Quaternary structure of Hb • Hb tetramer can be envisioned as composed of 2 identical dimers (αβ)1 and (αβ)2. • The 2 polyp chains in each dimer held tightly together primarily by hydrophobic interactions (in this case hydrophobic aa residues are localized not only in interior of molecule but also in a region on surface of each subunit. Interchain hydrophobic interactions form strong associations b/w α- and β-subunits in dimers) • Ionic and H-bonds also occur b/w members of the dimer • The two dimers, in contrast, are able to move wrt each other, being held primarily by polar bonds. The weaker interactions b/w these mobile dimers result in the 2 dimers occupying different relative positions in deoxy-Hb as compared with oxy-Hb
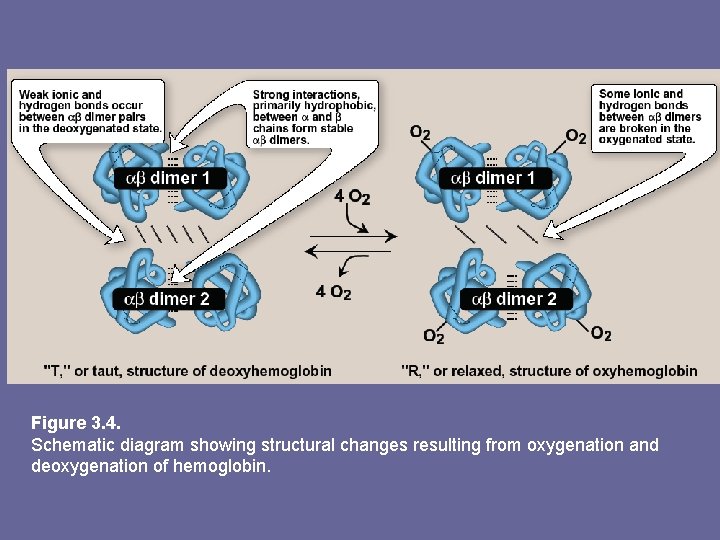
Figure 3. 4. Schematic diagram showing structural changes resulting from oxygenation and deoxygenation of hemoglobin.

a) T-form: • • • deoxy form of Hb “T”, or taut (tense) form. The 2 αβ dimers interact through a network of ionic and H-bonds constrain movement of polyp chains Low oxygen-affinity form of Hb b) R-form: – – – Binding of oxygen to Hb causes rupture of some of the ionic and H-bonds b/w αβ dimers This leads to a structure called “R” or relaxed form, here polyp chains have more freedom of movement R-form is high oxygen-affinity form of Hb

D. Binding of oxygen to myoglobin and Hb • • • Myoglobin can bind 1 O 2 molecule, it contains only 1 heme group Hb can bind 4 O 2 molecules, one at each of its 4 heme groups Degree of saturation (Y) of these oxygenbinding sites on all myoglobin or Hb molecules can vary b/w zero (all sites are empty) and 100% (all sites are full)

1. Oxygen dissociation curve • A plot of Y measured at different p. O 2 • Curves for myoglobin & Hb show important differences. Myoglobin has a higher oxygen affinity than Hb. Partial pressure of oxygen needed to achieve half-saturation of binding sites (P 50) is ~ 1 mm Hg for myoglobin & 26 mm Hg for Hb • Note: the higher the oxygen affinity (i. e. , the more tightly oxygen binds), the lower P 50

Figure 3. 5 Oxygen dissociation curves for myoglobin and hemoglobin.

a. Myoglobin • • • The oxygen dissociation curve for myoglobin has a hyperbolic shape. This reflects myoglobin reversibly binds a single molecule of oxygen Thus, oxygenated (Mb. O 2) and deoxygenated (Mb) exist in a simple equilibrium: Mb + O 2 ↔ Mb. O 2 Mb is designed to bind oxygen released by Hb at the low p. O 2 found in muscles. Mb releases oxygen within muscle cell in response to oxygen demand

b. Hb • • • The oxygen dissociation curve for Hb is sigmoidal in shape. This reflects that subunits cooperate in binding oxygen. Cooperative binding of O 2 by the 4 subunits of Hb means binding of O 2 to one heme group increases the oxygen affinity of remaining heme groups in the same Hb molecule, this effect is heme-heme interaction Although binding of 1 st O 2 is difficult, subsequent binding of O 2 occurs with high affinity, shown by steep upward curve in the region 20 -30 mm Hg
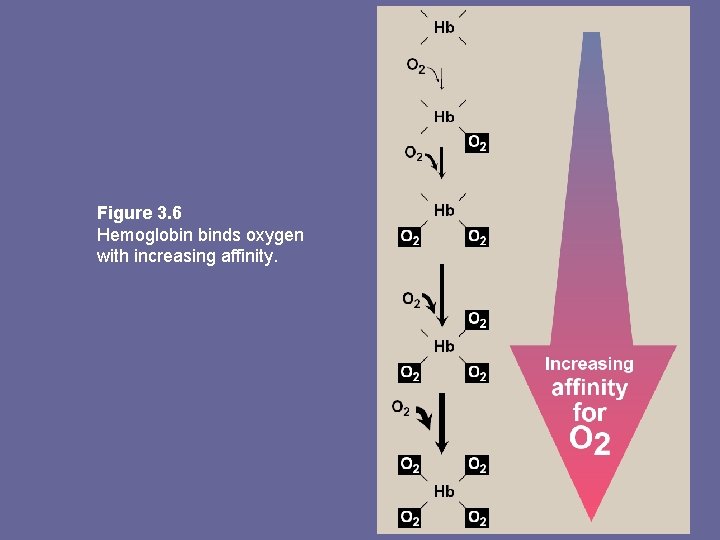
Figure 3. 6 Hemoglobin binds oxygen with increasing affinity.

E. Allosteric effects • Ability of Hb to reversibly bind oxygen is affected by p. O 2 (through heme-heme interaction), p. H of environ. p. CO 2 and availability of 2, 3 -bisphoglycerate. • Collectively called allosteric (“other site”) effectors, as their interaction at one site on Hb molecule affects binding of oxygen to heme groups at other locations on the molecule • Binding of oxygen to myoglobin is not influenced by allosteric effectors of Hb 1. Heme-heme interactions: sigmoidal oxygen-binding curve reflects specific structural changes that are initiated at one heme and transmitted to other heme groups in Hb tetramer. Net effect affinity of Hb for last oxygen ~ 300 x greater than affinity for 1 st oxygen

a. Loading and unloading oxygen: cooperative binding of oxygen allows Hb deliver more oxygen to tissues in response to relatively small changes in p. O 2. Figure 3. 5 indicates p. O 2 in alveoli of lung and capillaries of tissues - e. g. , in lung conc. oxygen is high and Hb becomes saturated (loaded) with oxygen. - in peripheral tissues, oxy-Hb releases (unloads) much of its oxygen for use in oxidative metabolism b. Significance of sigmoidal O 2 -dissociation curve: Steep slope of O 2 dissociation curve over the range of oxygen conc. b/w lungs and tissues permits Hb to carry and deliver oxygen efficiently from sites of high to sites of low p. O 2 A molecule with hyperbolic O 2 -dissociation curve, e. g. myoglobin could not achieve the same thing. Instead, it would have max affinity for oxygen throughout this oxygen pressure would deliver no oxygen to tissues
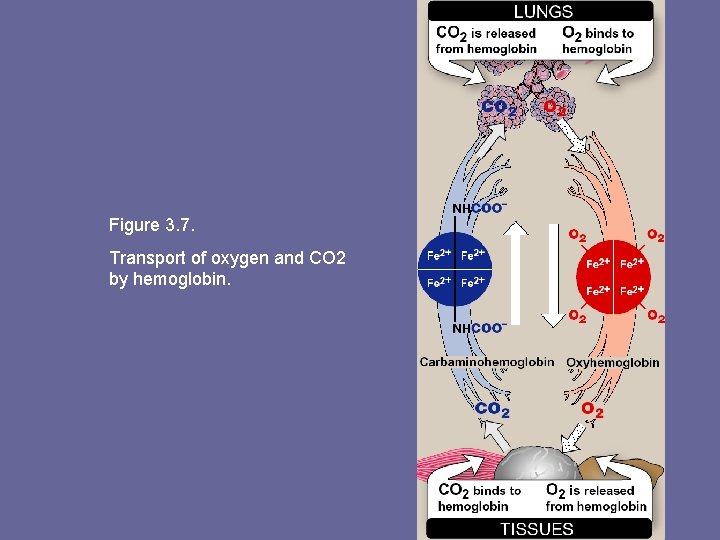
Figure 3. 7. Transport of oxygen and CO 2 by hemoglobin.

2. Bohr effect: – – – Release of oxygen from Hb is enhanced when p. H is lowered or when Hb is in pressure of an increased p. CO 2. Both result in decreased oxygen affinity shift to the right in O 2 -dissociation curve. This change in oxygen binding is called Bohr effect. Conversely, raising p. H or lowering conc. of CO 2 greater affinity for oxygen, and a shift to the left in O 2 -dissociation curve.
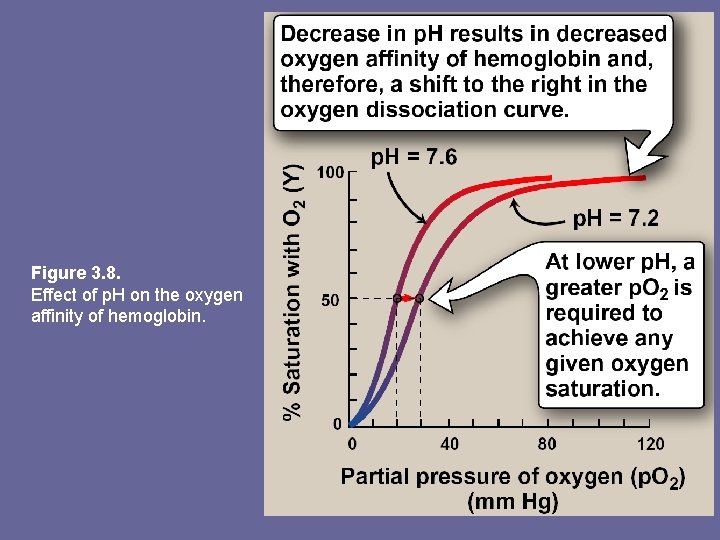
Figure 3. 8. Effect of p. H on the oxygen affinity of hemoglobin.
a. Source of the protons that lower the p. H: • Conc. of both CO 2 and H+ in capillaries of metabolically active tissues is higher than that observed in capillaries of lung, where CO 2 is released into expired air • Note: organic acids e. g. , lactic acid, are produced during anaerobic metabolism in rapidly contracting muscle • In tissues, CO 2 is converted by carbonic anhydrase to carbonic acid, CO 2 + H 2 O ↔ H 2 CO 3 which spontaneously loses a proton becoming bicarbonate, the major blood buffer H 2 CO 3 ↔ H+ + HCO 3 • The proton produced contributes to lowering p. H. This differential p. H gradient (lungs having higher, tissues lower p. H) favors unloading oxygen in peripheral tissues, and loading of oxygen in lung. • Thus, oxygen affinity of Hb responds to small shifts in p. H b/w lungs and oxygen-consuming tissues, making Hb a more efficient transporter of oxygen.

b. Mechanism of the Bohr effect: Deoxy form of Hb has a greater affinity for protons than does oxy. Hb. This fact is caused by ionizable groups, e. g. , N-terminal αamino groups, & specific His side chains that have higher p. Ka’s in deoxy-Hb than in oxy-Hb. An increase in conc. of protons causes these groups to become protonated (charged) and able to form ionic bonds (a. k. a salt bridges), which stabilize deoxy form of Hb, producing a decrease in oxygen affinity Bohr effect schematically: Hb. O 2 (oxy-Hb) + H+ ↔ Hb. H (deoxy-Hb) + O 2 where an increase in protons (or a lower p. O 2) shifts equilibrium to right, whereas an increase in p. O 2 (or decrease in protons) shifts equilibrium to left
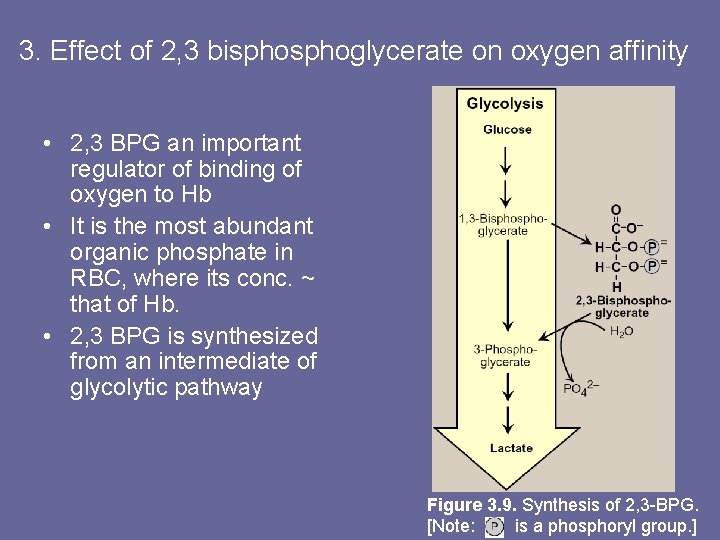
3. Effect of 2, 3 bisphoglycerate on oxygen affinity • 2, 3 BPG an important regulator of binding of oxygen to Hb • It is the most abundant organic phosphate in RBC, where its conc. ~ that of Hb. • 2, 3 BPG is synthesized from an intermediate of glycolytic pathway Figure 3. 9. Synthesis of 2, 3 -BPG. [Note: is a phosphoryl group. ]
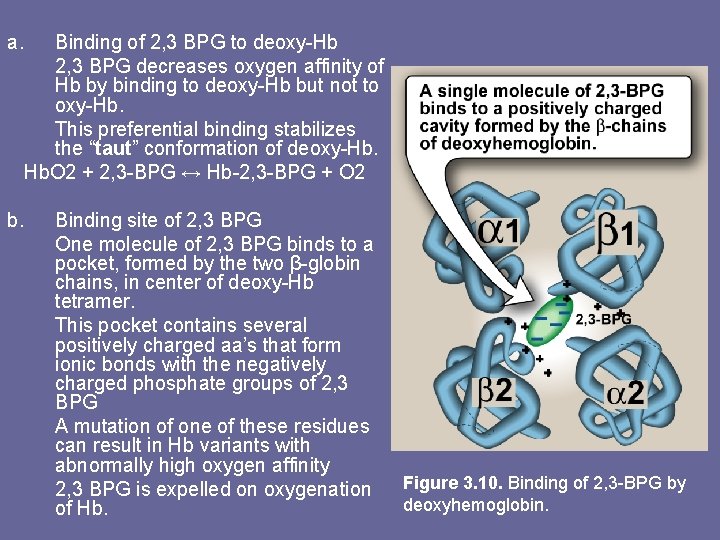
a. b. Binding of 2, 3 BPG to deoxy-Hb 2, 3 BPG decreases oxygen affinity of Hb by binding to deoxy-Hb but not to oxy-Hb. This preferential binding stabilizes the “taut” conformation of deoxy-Hb. O 2 + 2, 3 -BPG ↔ Hb-2, 3 -BPG + O 2 Binding site of 2, 3 BPG One molecule of 2, 3 BPG binds to a pocket, formed by the two β-globin chains, in center of deoxy-Hb tetramer. This pocket contains several positively charged aa’s that form ionic bonds with the negatively charged phosphate groups of 2, 3 BPG A mutation of one of these residues can result in Hb variants with abnormally high oxygen affinity 2, 3 BPG is expelled on oxygenation of Hb. Figure 3. 10. Binding of 2, 3 -BPG by deoxyhemoglobin.
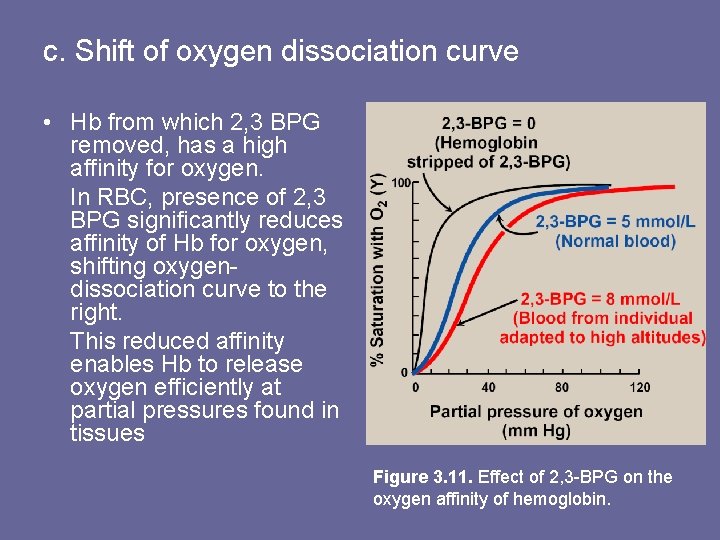
c. Shift of oxygen dissociation curve • Hb from which 2, 3 BPG removed, has a high affinity for oxygen. In RBC, presence of 2, 3 BPG significantly reduces affinity of Hb for oxygen, shifting oxygendissociation curve to the right. This reduced affinity enables Hb to release oxygen efficiently at partial pressures found in tissues Figure 3. 11. Effect of 2, 3 -BPG on the oxygen affinity of hemoglobin.

d. Response of 2, 3 -BPG levels to chronic hypoxia or anemia • Conc. of 2, 3 BPG in RBC increases in response to chronic hypoxia, e. g. , that observed in obstructive pulmonary emphysema, or at high altitudes, where Hb may have difficulty receiving sufficient oxygen. Intracellular 2, 3 BPG also elevated in chronic anemia in which fewer than normal RBCs are available to supply body’s oxygen needs. Elevated 2, 3 BPG levels lower oxygen affinity of HB, permitting greater unloading of oxygen in capillaries of tissues
e. Role of 2, 3 BPG in transfused blood: • 2, 3 BPG is essential for normal oxygen transport function of Hb. • e. g. , storing blood in acid-citrate-dextrose decrease of 2, 3 BPG in RBCs. Such blood displays an abnormally high oxygen affinity, and fails to unload its bound oxygen properly in the tissues. • Hb deficient in 2, 3 BPG thus acts as an oxygen “trap” rather than as an oxygen transport system.

• Transfused RBCs are able to restore depleted supplies of 2, 3 BPG in 24 -48 h. However, severely ill patients may be seriously compromised if transfused with large quantities of such 2, 3 BPG –”stripped” blood. • Decrease in 2, 3 BPG can be prevented by adding substrates e. g. , inosine (hypoxanthine -ribose) to storage medium. • Inosine, uncharged molecule can enter RBC, its ribose moiety is released, phosphorylated, and enters hexose mosophosphate pathway, eventually converted to 2, 3 BPG

4. Binding of CO 2: • Most CO 2 produced in metabolism is hydrated and transported as bicarbonate ion. • However, some CO 2 is carried as carbamate bound to uncharged α-amino groups of Hb (carbamino-Hb): Hb-NH 2 + CO 2 ↔ Hb-NH-COO- + H+ • Binding of CO 2 stabilizes T (taut) or deoxy form of Hb, resulting in decrease in its affinity for oxygen. In lungs, CO 2 dissociates from Hb, released in breath.

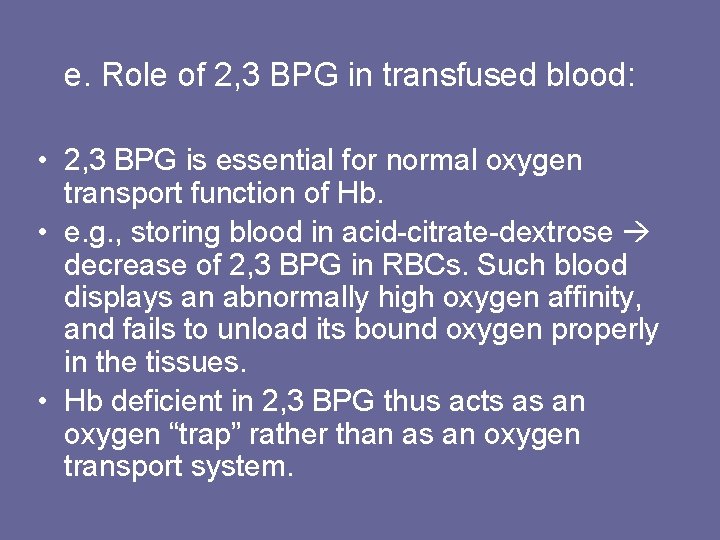
5. Binding of CO: • CO binds tightly (but reversibly) to Hb iron carbon monoxyhemoglobin (Hb. CO). • When CO binds to one or more of the 4 heme sites, Hb shifts to relaxed conformation, causing remaining sites bind oxygen with high affinity. • This shifts oxygen saturation curve to the left, and changes normal sigmoidal shape toward a hyperbola. • As a result affected Hb is unable to release oxygen to tissues • Affinity of Hb to CO is 220 x greater than for oxygen.
Figure 3. 12. Effect of carbon monoxide on the oxygen affinity of hemoglobin. CO-Hb = carbon monoxyhemoglobin.

• Even minute quantities of CO in environ. can produce toxic conc’s of Hb. CO in blood. • CO toxicity appears to result from a combination of tissue hypoxia and direct COmediated damage at cellular level. • CO poisoning is treated with 100% oxygen therapy, which facilitates dissociation of CO from Hb.
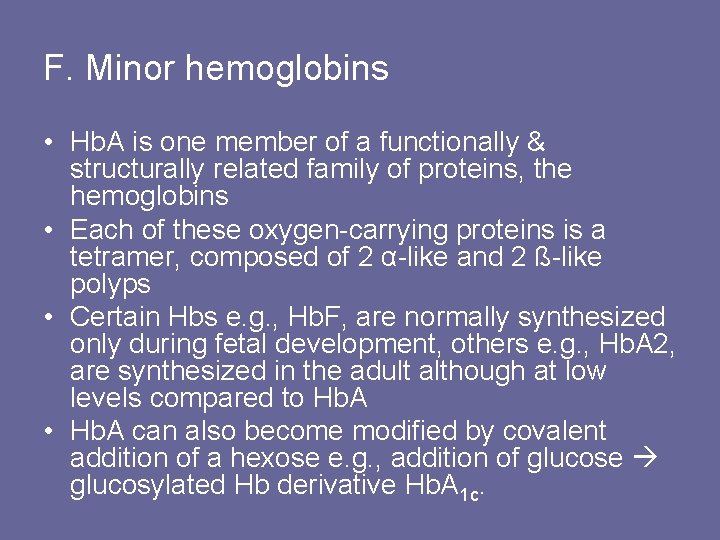
F. Minor hemoglobins • Hb. A is one member of a functionally & structurally related family of proteins, the hemoglobins • Each of these oxygen-carrying proteins is a tetramer, composed of 2 α-like and 2 ß-like polyps • Certain Hbs e. g. , Hb. F, are normally synthesized only during fetal development, others e. g. , Hb. A 2, are synthesized in the adult although at low levels compared to Hb. A • Hb. A can also become modified by covalent addition of a hexose e. g. , addition of glucose glucosylated Hb derivative Hb. A 1 c.
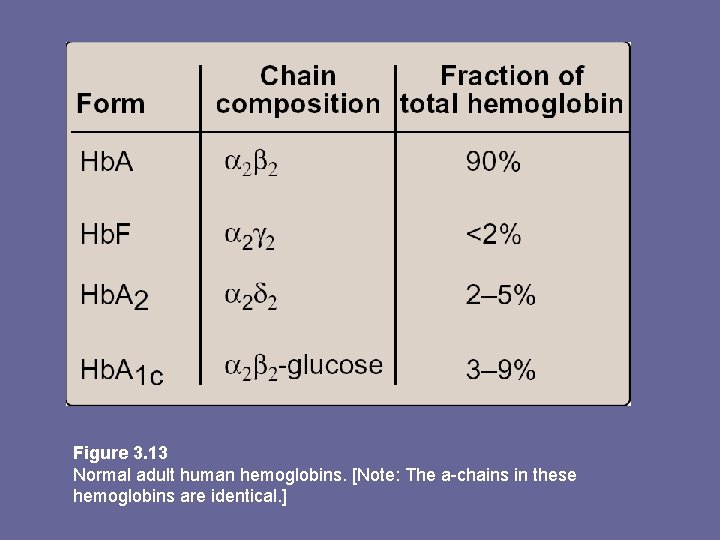
Figure 3. 13 Normal adult human hemoglobins. [Note: The a-chains in these hemoglobins are identical. ]

Hb. A 2: • Is a minor component of normal adult Hb, appearing ~ 12 wks after birth ~ 2% of total Hb. composed of α 2δ 2. Hb. A 1 c: • Under physiologic conditions, Hb. A slowly & non-enzymatically glycosylated • Extent of glycosylation dependent on plasma conc. of a particular hexose • Most abundant form of glycosylated Hb is Hb. A 1 c: has glucose residues attached predominantly to NH 2 groups of the N-terminal Val of ß-globin chains • Increased amounts found in RBCs of diabetes mellitus, as their Hb. A has contact with higher glucose concs during the 120 d lifetime of these cells
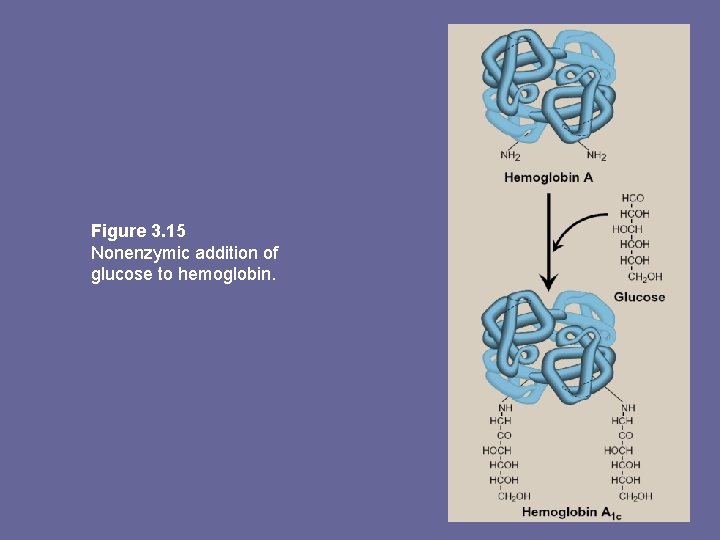
Figure 3. 15 Nonenzymic addition of glucose to hemoglobin.

Summary • Hb. A major adult Hb : α 2ß 2 held together by non-covalent interactions. Subunits occupy different relative positions in deoxy- & oxyhemoglobin • Deoxy- form = “T” or taut (tense) form. It has a constrained structure that limits movement of polyp chains • T form is low oxygen affinity form. • Binding of oxygen rupture of some ionic & H-bonds R (relaxed form), subunits have more freedom of movement • R form is high oxygen affinity form
• Oxygen dissociation curve for Hb is sigmoidal (that of myoglobin is hyperbolic) indicating subunits cooperate in binding oxygen i. e. , binding to one heme increases affinity of remaining heme groups in same Hb molecule • Hb’s ability to bind oxygen reversibly is affected by p. O 2, and availability of 2, 3 BPG e. g. , release of O 2 from Hb is enhance when p. H lowered or o. CO 2 increased (Bohr effect), e. g. , in exercising muscle, & oxygen dissociation curve of Hb is shifted to right. • To cope with long-term effects of chronic hypoxia or anemia, conc of 2, 3 BPG in RBCs increases. 2, 3 BPG binds Hb and decreases affinity to oxygen shifts curve to right • CO binds tightly (but reversibly) to Hb iron, carbon monoxy. Hb (Hb. CO) • Hemoglobinopathies are disorders of Hb caused by production of structurally abnormal or synthesis of insufficient quantities of normal Hb subunits, or rarely, both. • Hb. S disease, Hb. C disease, & thalassemia syndromes are examples and can have severe clinical consequences
- Slides: 44