Unit Eleven Solutions Solutions A solution is a




















- Slides: 42

Unit Eleven: Solutions

Solutions • A solution is a homogeneous mixture of two or more substances ▫ Homogeneous – uniform characteristics throughout ▫ Heterogeneous – different compositions; various throughout • Solutions have at least two components ▫ Solutes – the minority component ▫ Solvents – the majority component ▫ 75% isopropyl solution – solute is water, solvent is isopropyl ▫ 3% H 2 O 2 – solute is H 2 O 2, solvent is water

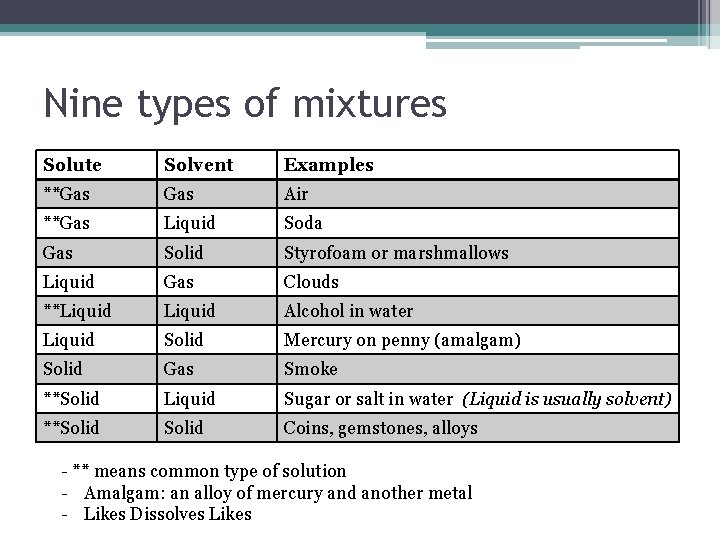
Nine types of mixtures Solute Solvent Examples **Gas Air **Gas Liquid Soda Gas Solid Styrofoam or marshmallows Liquid Gas Clouds **Liquid Alcohol in water Liquid Solid Mercury on penny (amalgam) Solid Gas Smoke **Solid Liquid Sugar or salt in water (Liquid is usually solvent) **Solid Coins, gemstones, alloys - ** means common type of solution - Amalgam: an alloy of mercury and another metal - Likes Dissolves Likes

Non-Solutions - Suspensions • Suspensions ▫ A mixture from which particles settle out upon standing ▫ Particles are larger than in a solution ▫ Suspended – temporarily out of school ▫ Examples: Italian Dressing, muddy water, orange juice with pulp

Non-Solutions - Colloids • Colloids ▫ A permanent mixture whose particles are smaller than in a suspension and larger than in a solution ▫ Particles will reflect light, cloudy appearance ▫ Do not settle out and cannot be filtered ▫ Milk, starch, dusty air, fog

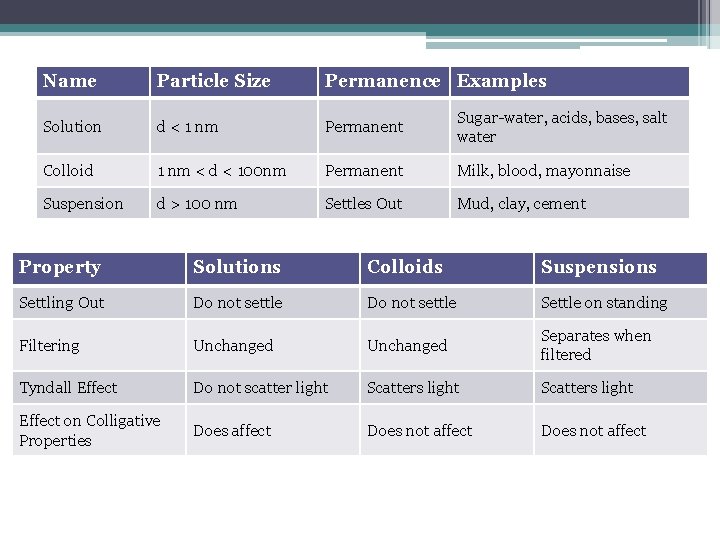
Name Particle Size Permanence Examples Solution d < 1 nm Permanent Sugar-water, acids, bases, salt water Colloid 1 nm < d < 100 nm Permanent Milk, blood, mayonnaise Suspension d > 100 nm Settles Out Mud, clay, cement Property Solutions Colloids Suspensions Settling Out Do not settle Settle on standing Filtering Unchanged Separates when filtered Tyndall Effect Do not scatter light Scatters light Effect on Colligative Properties Does affect Does not affect

Tyndall Effect Laser pointed through a solution, colloid, and suspension – what will happen?

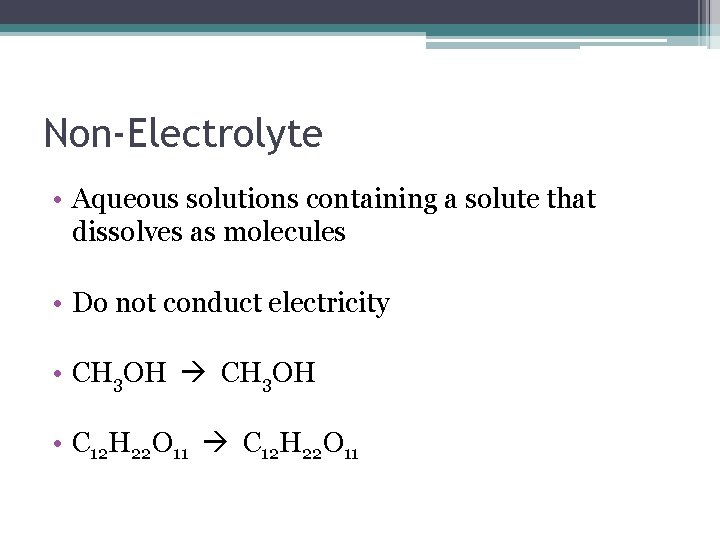
Non-Electrolyte • Aqueous solutions containing a solute that dissolves as molecules • Do not conduct electricity • CH 3 OH • C 12 H 22 O 11

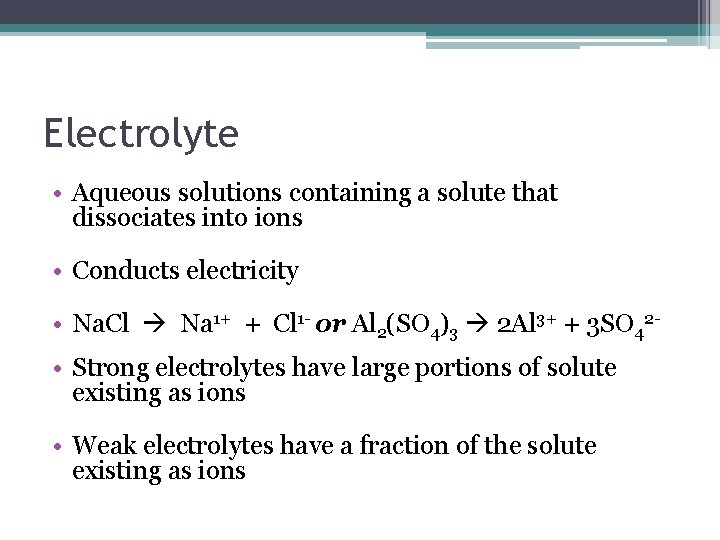
Electrolyte • Aqueous solutions containing a solute that dissociates into ions • Conducts electricity • Na. Cl Na 1+ + Cl 1 - or Al 2(SO 4)3 2 Al 3+ + 3 SO 42 • Strong electrolytes have large portions of solute existing as ions • Weak electrolytes have a fraction of the solute existing as ions

Solubility • Solubility is the amount of compound (usually in grams) that will dissolve in a certain amount of liquid • There are three types of solutions: ▫ Unsaturated ▫ Supersaturated

Solubility • Saturated Solutions ▫ Hold the maximum amount of solute under the solution conditions ▫ If additional solute is added to a saturated solution, it will not dissolve • Unsaturated Solutions ▫ Hold less than the maximum amount of solute under the solution conditions ▫ If additional solute is added to an unsaturated solution, it will dissolve • Supersaturated Solutions ▫ Hold more than the normal maximum amount of solute ▫ Any disturbance will precipitate the solute or make it come out of solution

Supersaturated Solution Example: Rock Candy

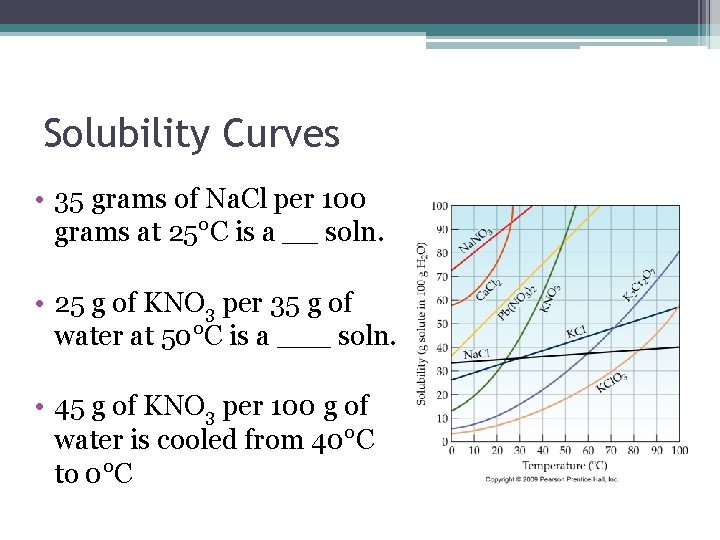
Solubility Curves • 35 grams of Na. Cl per 100 grams at 25°C is a __ soln. • 25 g of KNO 3 per 35 g of water at 50°C is a ___ soln. • 45 g of KNO 3 per 100 g of water is cooled from 40°C to 0°C

Solubility • Solubility depends on: ▫ Identity of solute and solvent �Like dissolves like -> polar dissolves polar and nonpolar dissolves nonpolar ▫ Temperature �For solids in liquids, solubility increases with increasing temperature �For gases in liquids, solubility decreases with increasing temperature

Solubility ▫ Pressure �For solids in liquids, a change in pressure has very little effect on the solubility �For gases in liquids, higher pressure increases the solubility of the gas in the liquid �When a can of soda is opened, there is less pressure so the gas is less soluble

• Worksheet One is due Friday (Tomorrow) • Worksheet Two is due Monday ▫ Skip Question 10 ▫ Questions 3, 4, and 5 you cannot answer until Friday’s notes

Solubility • Rate of solution – ▫ How fast a substance dissolves and how quickly the substance goes into solution ▫ Factors that increase the rate of solution for solids �Decrease particle size �Stirring �Increase temperature

Hydration versus Solvation • Hydration ▫ A solute is dissolved by water molecules attaching to ions and moving them into solution • Solvation ▫ Process of molecules of a solvent moving molecules or ions into solution

Concentrations • Concentrations – the amount of solute in a solution ▫ A dilute solution is one containing small amounts of solute relative to solvent ▫ A concentrated solution is one containing large amounts of solute relative to solvent ▫ Mass percent, molarity, and molality

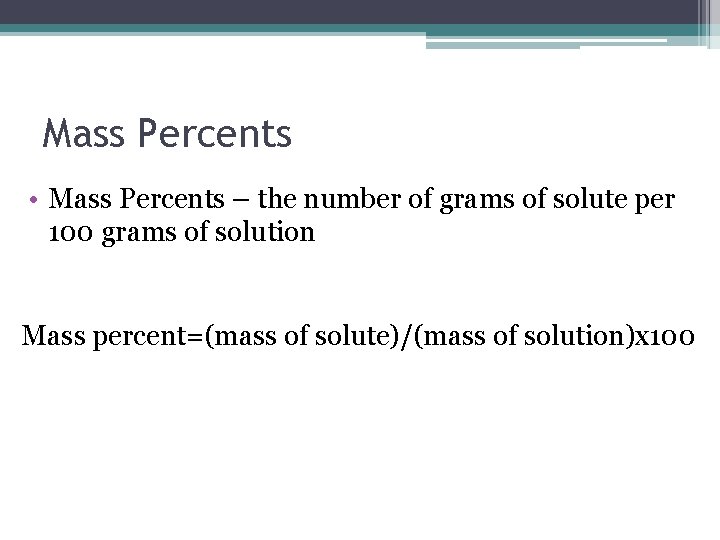
Mass Percents • Mass Percents – the number of grams of solute per 100 grams of solution Mass percent=(mass of solute)/(mass of solution)x 100

Practice Problems • Calculate the mass percent of Na. Cl in a solution containing 15. 3 grams of Na. Cl and 155. 0 grams H 2 O. • Calculate the mass percent of a solution containing 27. 5 grams C 2 H 6 O and 175 m. L of H 2 O • A soft drink contains 11. 5% sucrose by mass. What volume of soft drink solution, in m. L, contains 85. 2 grams of sucrose?

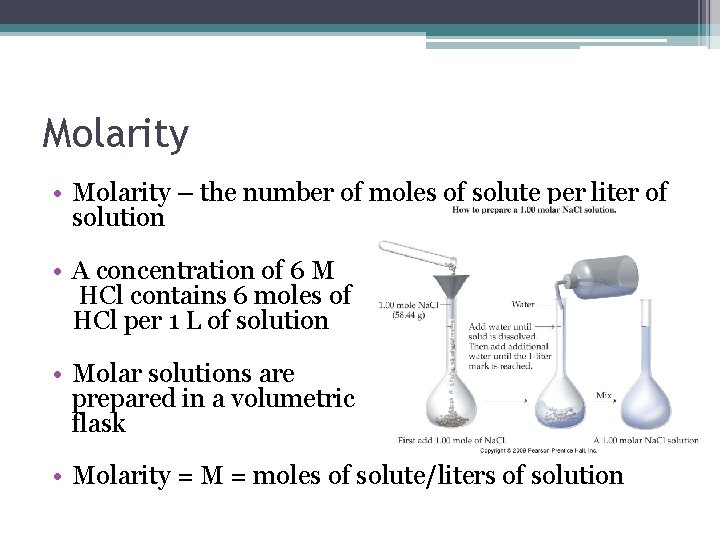
Molarity • Molarity – the number of moles of solute per liter of solution • A concentration of 6 M HCl contains 6 moles of HCl per 1 L of solution • Molar solutions are prepared in a volumetric flask • Molarity = M = moles of solute/liters of solution

Practice Problems • Calculate the molarity of a sucrose solution made with 1. 58 moles of solute diluted to a total volume of 5. 0 L of solution. • Calculate the molarity of a solution made by putting 15. 5 grams of Na. Cl into a beaker and adding water to make 1. 50 L of Na. Cl solution.

Practice problems • 6. 7 grams of NH 4 Cl is dissolved in enough water to make 803 m. L of solution. What is the molarity of the solution. • How many grams of Na. OH are needed to make 500. 0 m. L of a 1. 00 M solution? • What volume of a 1. 0 M Na. NO 3 solution can be prepared from 170. 0 grams of Na. NO 3?

Solution Dilutions • Most solutions are bought as stock solutions, however most labs need diluted solutions. • We use M 1 V 1=M 2 V 2

Practice Problems • A laboratory procedure calls for 5. 00 L of a 1. 50 M KCl solution. How should you prepare this solution from a 12. 0 M stock solution? • To what volume should you dilute 0. 100 L of a 15 M Na. OH solution to obtain a 1. 0 M Na. OH solution?

Practice Problems • How much 6. 0 M Na. NO 3 solution should be used to make 0. 585 L of a 1. 2 M Na. NO 3 solution?

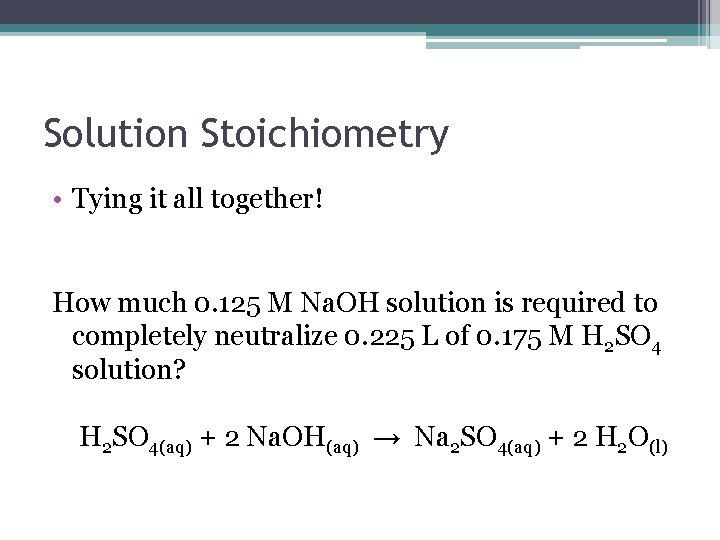
Solution Stoichiometry • Tying it all together! How much 0. 125 M Na. OH solution is required to completely neutralize 0. 225 L of 0. 175 M H 2 SO 4 solution? H 2 SO 4(aq) + 2 Na. OH(aq) → Na 2 SO 4(aq) + 2 H 2 O(l)

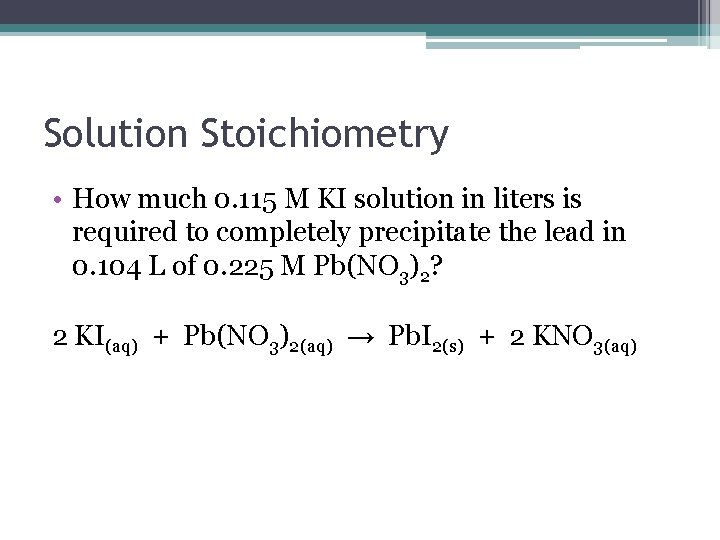
Solution Stoichiometry • How much 0. 115 M KI solution in liters is required to completely precipitate the lead in 0. 104 L of 0. 225 M Pb(NO 3)2? 2 KI(aq) + Pb(NO 3)2(aq) → Pb. I 2(s) + 2 KNO 3(aq)

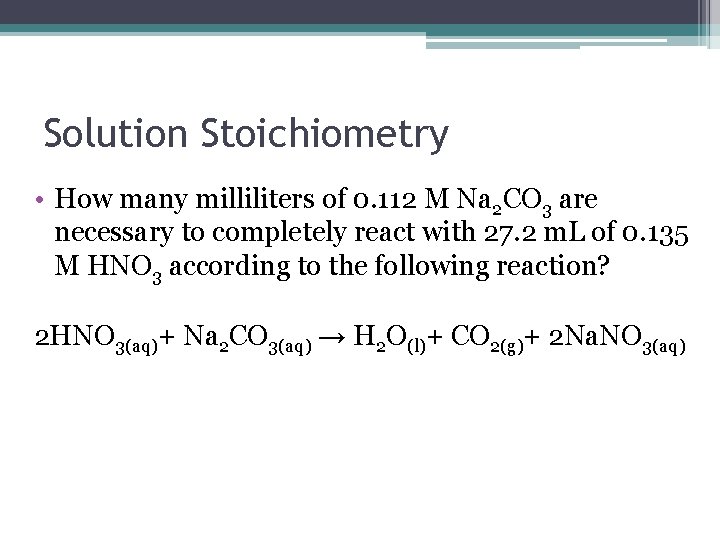
Solution Stoichiometry • How many milliliters of 0. 112 M Na 2 CO 3 are necessary to completely react with 27. 2 m. L of 0. 135 M HNO 3 according to the following reaction? 2 HNO 3(aq)+ Na 2 CO 3(aq) → H 2 O(l)+ CO 2(g)+ 2 Na. NO 3(aq)


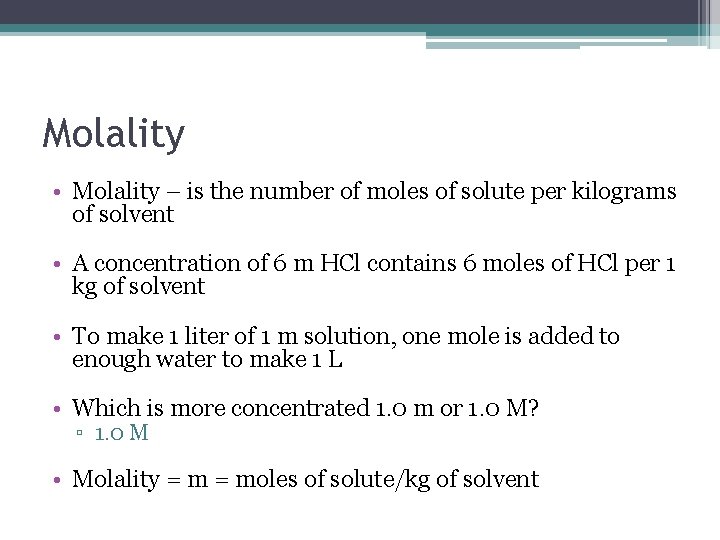
Molality • Molality – is the number of moles of solute per kilograms of solvent • A concentration of 6 m HCl contains 6 moles of HCl per 1 kg of solvent • To make 1 liter of 1 m solution, one mole is added to enough water to make 1 L • Which is more concentrated 1. 0 m or 1. 0 M? ▫ 1. 0 M • Molality = moles of solute/kg of solvent

Practice Problems • What is the molality of the solution if 5. 0 grams of KI are in 500. 0 grams of water? • How many grams of calcium nitrate (Ca(NO 3)2) must be added to 20. 0 grams of water to make a 2. 5 m solution?

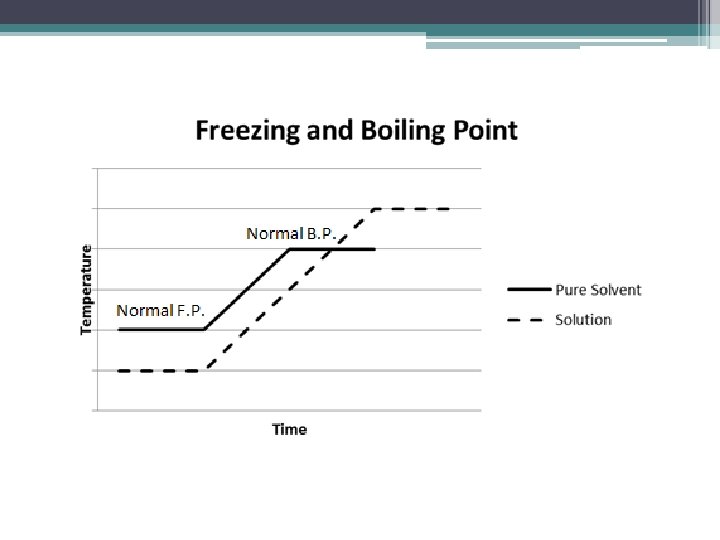
Colligative Properties • A property that depends on the number of solute particles and not the type of solute particles • How much you have not what you have • Two types ▫ Freezing point depression ▫ Boiling point elevation ▫ These depend on quantity of solute not type of solute


Colligative Properties • Freezing point depression – difference in temperature between the freezing point of a solution and the freezing point of the pure solvent ▫ Solute disrupts the formation of the orderly pattern thus requiring more energy �Solution freezes at a lower temperature than the pure solvent ▫ Magnitude is proportional to the number of solute particles dissolved

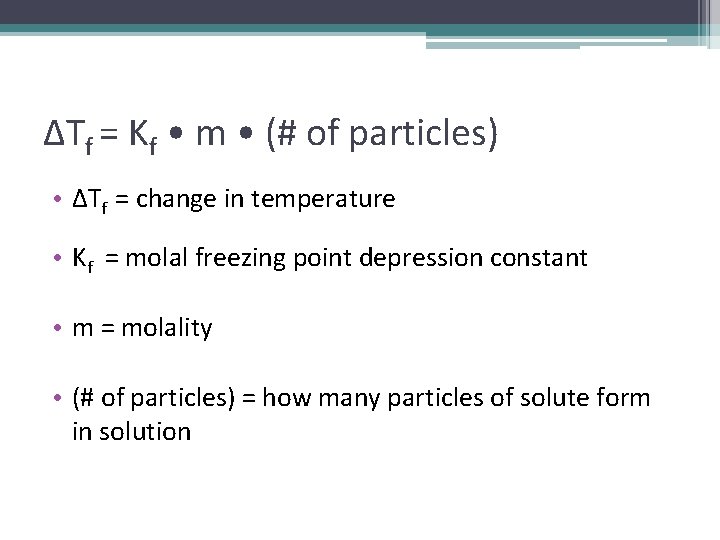
ΔTf = Kf • m • (# of particles) • ΔTf = change in temperature • Kf = molal freezing point depression constant • m = molality • (# of particles) = how many particles of solute form in solution

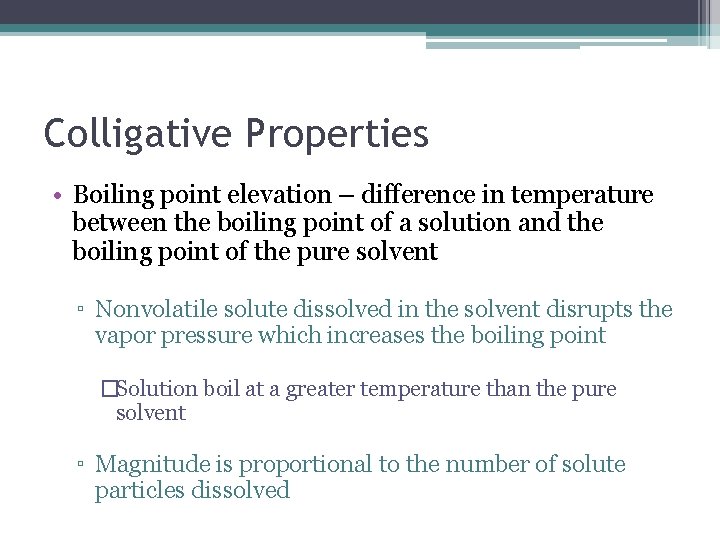
Colligative Properties • Boiling point elevation – difference in temperature between the boiling point of a solution and the boiling point of the pure solvent ▫ Nonvolatile solute dissolved in the solvent disrupts the vapor pressure which increases the boiling point �Solution boil at a greater temperature than the pure solvent ▫ Magnitude is proportional to the number of solute particles dissolved

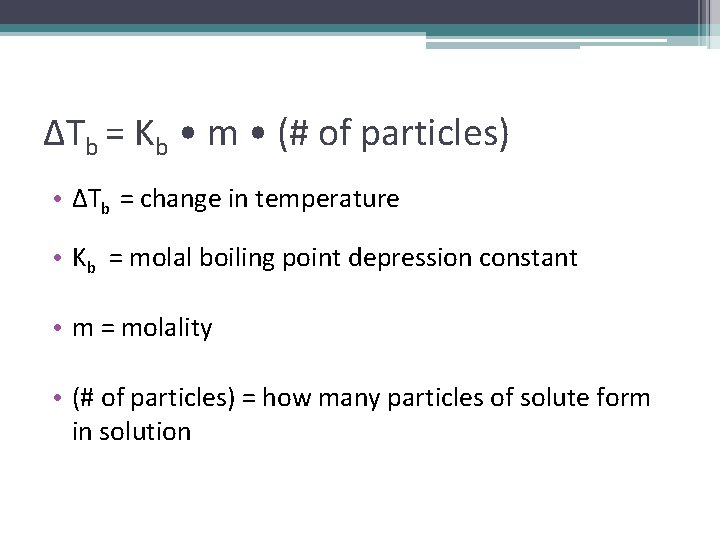
ΔTb = Kb • m • (# of particles) • ΔTb = change in temperature • Kb = molal boiling point depression constant • m = molality • (# of particles) = how many particles of solute form in solution

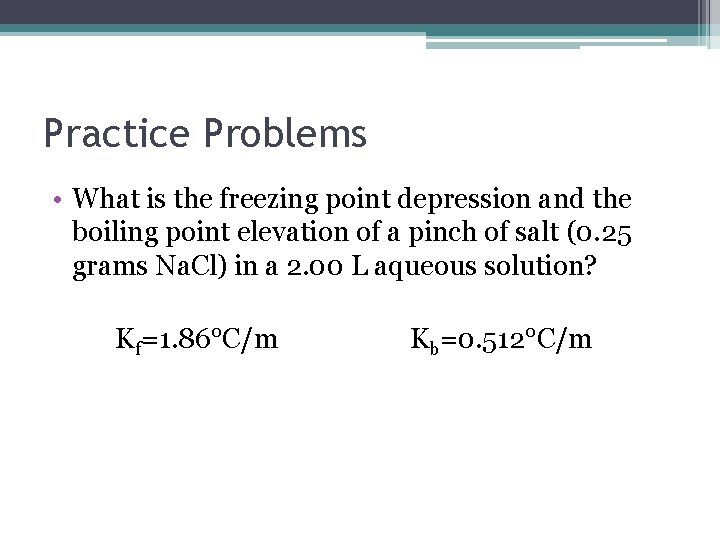
Practice Problems • What is the freezing point depression and the boiling point elevation of a pinch of salt (0. 25 grams Na. Cl) in a 2. 00 L aqueous solution? Kf=1. 86°C/m Kb=0. 512°C/m

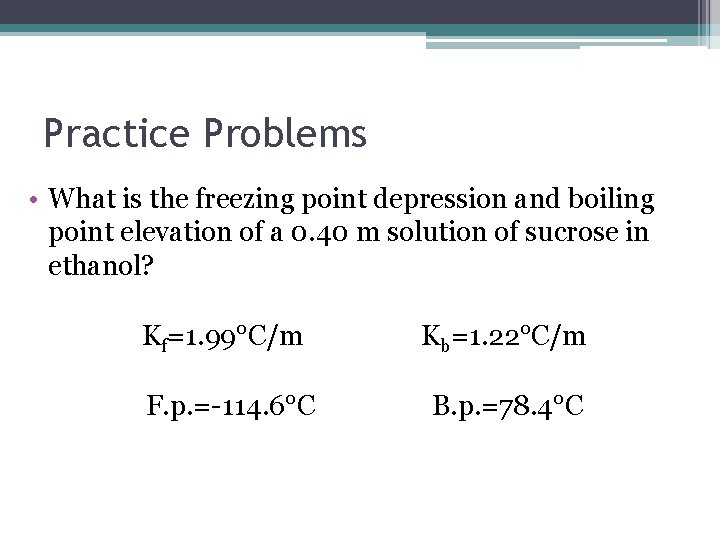
Practice Problems • What is the freezing point depression and boiling point elevation of a 0. 40 m solution of sucrose in ethanol? Kf=1. 99°C/m Kb=1. 22°C/m F. p. =-114. 6°C B. p. =78. 4°C

• Unit Eleven Test Tomorrow (Friday March 8 th) • Worksheet Five due Tomorrow • Gizmo’s due Tomorrow • Lab Report Revisions due Tuesday ▫ Will have optional computer time on Monday