Unit Electrochemistry Oxidation Numbers and Half Reactions Redox
Unit: Electrochemistry Oxidation Numbers and Half. Reactions
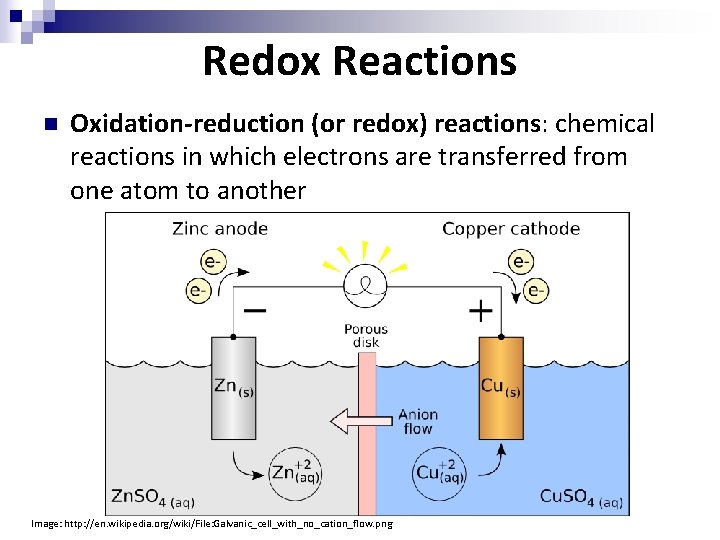
Redox Reactions n Oxidation-reduction (or redox) reactions: chemical reactions in which electrons are transferred from one atom to another Image: http: //en. wikipedia. org/wiki/File: Galvanic_cell_with_no_cation_flow. png

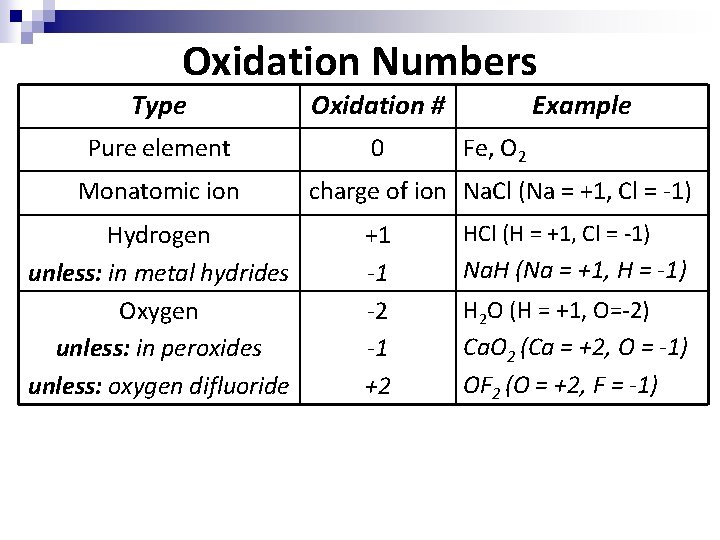
Oxidation Numbers n n In order to keep track of where the electrons are moving to and from, we use a bookkeeping system called “oxidation numbers”. An oxidation number is the apparent charge of the atom in the bond.
Oxidation Numbers Type Oxidation # Pure element 0 Monatomic ion Hydrogen unless: in metal hydrides Oxygen unless: in peroxides unless: oxygen difluoride Example Fe, O 2 charge of ion Na. Cl (Na = +1, Cl = -1) +1 -1 -2 -1 +2 HCl (H = +1, Cl = -1) Na. H (Na = +1, H = -1) H 2 O (H = +1, O=-2) Ca. O 2 (Ca = +2, O = -1) OF 2 (O = +2, F = -1)
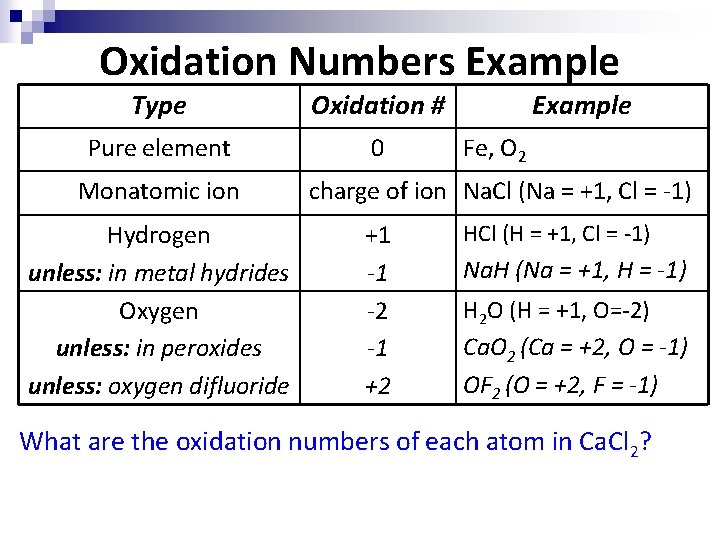
Oxidation Numbers Example Type Oxidation # Pure element 0 Monatomic ion Hydrogen unless: in metal hydrides Oxygen unless: in peroxides unless: oxygen difluoride Example Fe, O 2 charge of ion Na. Cl (Na = +1, Cl = -1) +1 -1 -2 -1 +2 HCl (H = +1, Cl = -1) Na. H (Na = +1, H = -1) H 2 O (H = +1, O=-2) Ca. O 2 (Ca = +2, O = -1) OF 2 (O = +2, F = -1) What are the oxidation numbers of each atom in Ca. Cl 2?
Oxidation Numbers Oxidation numbers must follow conservation of charge – the charges of the individual atoms must add up to the overall charge of the molecule or ion
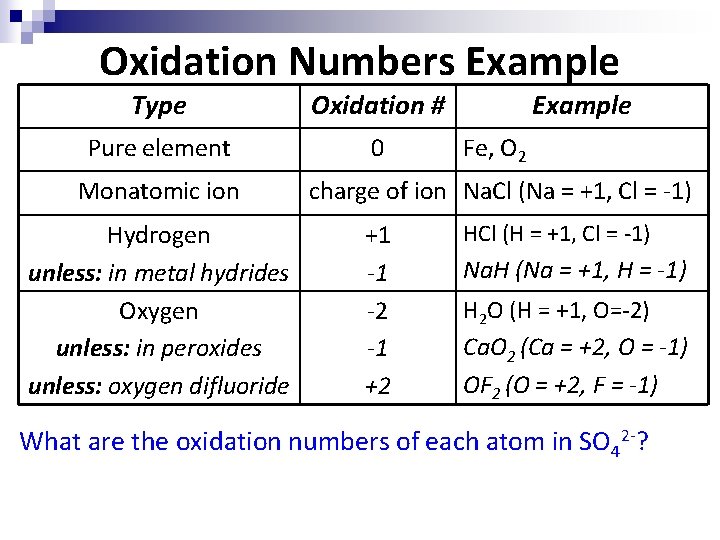
Oxidation Numbers Example Type Oxidation # Pure element 0 Monatomic ion Hydrogen unless: in metal hydrides Oxygen unless: in peroxides unless: oxygen difluoride Example Fe, O 2 charge of ion Na. Cl (Na = +1, Cl = -1) +1 -1 -2 -1 +2 HCl (H = +1, Cl = -1) Na. H (Na = +1, H = -1) H 2 O (H = +1, O=-2) Ca. O 2 (Ca = +2, O = -1) OF 2 (O = +2, F = -1) What are the oxidation numbers of each atom in SO 42 -?

Oxidation Numbers Example Type Oxidation # Pure element 0 Monatomic ion Hydrogen unless: in metal hydrides Oxygen unless: in peroxides unless: oxygen difluoride Example Fe, O 2 charge of ion Na. Cl (Na = +1, Cl = -1) +1 -1 -2 -1 +2 HCl (H = +1, Cl = -1) Na. H (Na = +1, H = -1) H 2 O (H = +1, O=-2) Ca. O 2 (Ca = +2, O = -1) OF 2 (O = +2, F = -1) What are the oxidation numbers of each atom in Na. NO 3?

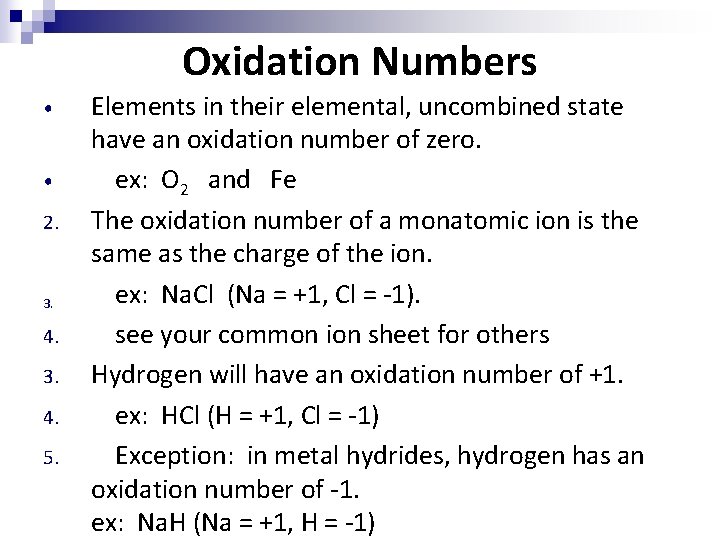
Oxidation Numbers • • 2. 3. 4. 3. 4. 5. Elements in their elemental, uncombined state have an oxidation number of zero. ex: O 2 and Fe The oxidation number of a monatomic ion is the same as the charge of the ion. ex: Na. Cl (Na = +1, Cl = -1). see your common ion sheet for others Hydrogen will have an oxidation number of +1. ex: HCl (H = +1, Cl = -1) Exception: in metal hydrides, hydrogen has an oxidation number of -1. ex: Na. H (Na = +1, H = -1)
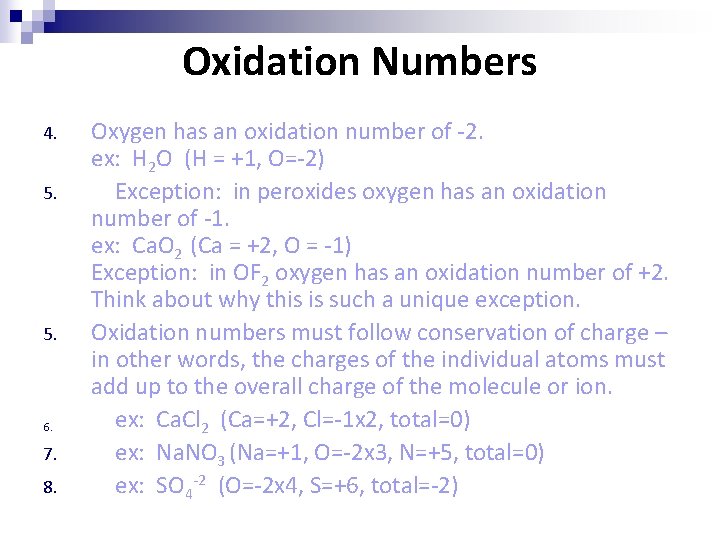
Oxidation Numbers 4. 5. 6. 7. 8. Oxygen has an oxidation number of -2. ex: H 2 O (H = +1, O=-2) Exception: in peroxides oxygen has an oxidation number of -1. ex: Ca. O 2 (Ca = +2, O = -1) Exception: in OF 2 oxygen has an oxidation number of +2. Think about why this is such a unique exception. Oxidation numbers must follow conservation of charge – in other words, the charges of the individual atoms must add up to the overall charge of the molecule or ion. ex: Ca. Cl 2 (Ca=+2, Cl=-1 x 2, total=0) ex: Na. NO 3 (Na=+1, O=-2 x 3, N=+5, total=0) ex: SO 4 -2 (O=-2 x 4, S=+6, total=-2)
- Slides: 11