Unit E Acids and bases Quantitative problem solving

Unit E Acids and bases: Quantitative problem solving
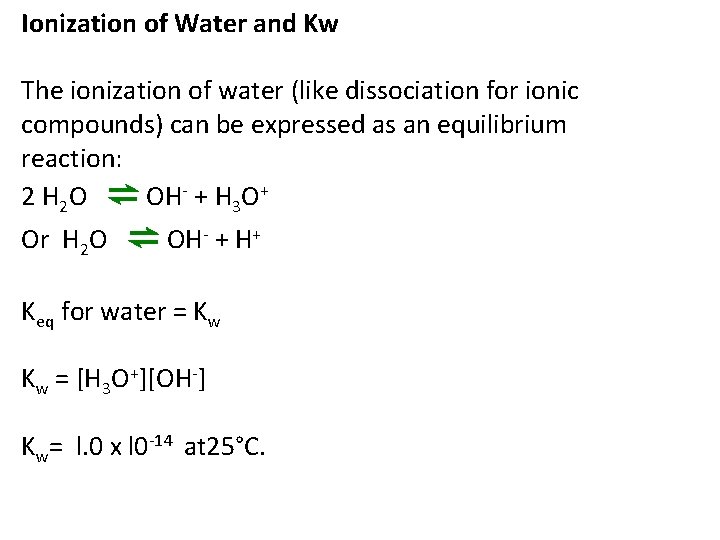
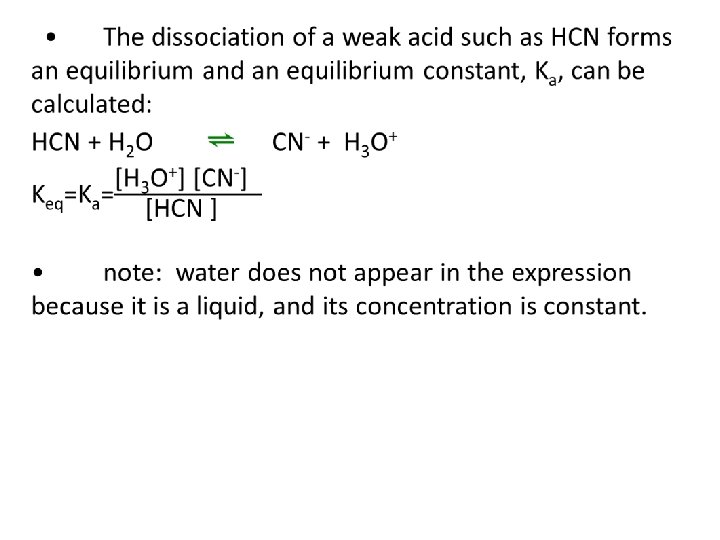
Ionization of Water and Kw The ionization of water (like dissociation for ionic compounds) can be expressed as an equilibrium reaction: 2 H 2 O ⇌ OH- + H 3 O+ Or H 2 O ⇌ OH- + H+ Keq for water = Kw Kw = [H 3 O+][OH-] Kw= l. 0 x l 0 -14 at 25°C.
![Quick Question • In a neutral solution (when [H+] and [OH-] are equal), what Quick Question • In a neutral solution (when [H+] and [OH-] are equal), what](http://slidetodoc.com/presentation_image_h2/888d6c4265683ad0dcac78f335e7e2ed/image-3.jpg)
Quick Question • In a neutral solution (when [H+] and [OH-] are equal), what is the [H+] and [OH-]?
![A rule to remember…. if [H+] ˃[OH-], the solution is ACIDIC. if [H+] ˂ A rule to remember…. if [H+] ˃[OH-], the solution is ACIDIC. if [H+] ˂](http://slidetodoc.com/presentation_image_h2/888d6c4265683ad0dcac78f335e7e2ed/image-4.jpg)
A rule to remember…. if [H+] ˃[OH-], the solution is ACIDIC. if [H+] ˂ [OH-], the solution is Basic

• the ionization of water is an endothermic process. H 2 O + energy ⇌ OH- + H+ Predict: which way will the equilibrium shift if the Temperature is increased? How will this affect Kw? • Therefore , Kw will increase as the temperature increases because the equilibrium shifts right.
![Ion Concentration Calculations Example 1: • Find the [H 3 O+] in 0. 20 Ion Concentration Calculations Example 1: • Find the [H 3 O+] in 0. 20](http://slidetodoc.com/presentation_image_h2/888d6c4265683ad0dcac78f335e7e2ed/image-6.jpg)
Ion Concentration Calculations Example 1: • Find the [H 3 O+] in 0. 20 M HCl. Example 2: • Find the [H 3 O+] in 0. 20 M KOH. Example 3: • Find the [ OH-] in 3. 0 M HCl.

ASSIGNMENT workbook
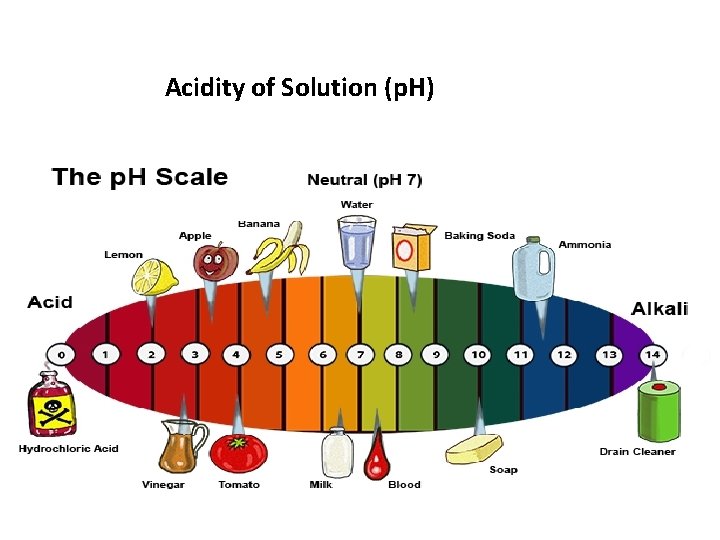
Acidity of Solution (p. H)
![PH • the [H 3 O+] can vary a great deal!! in a neutral PH • the [H 3 O+] can vary a great deal!! in a neutral](http://slidetodoc.com/presentation_image_h2/888d6c4265683ad0dcac78f335e7e2ed/image-9.jpg)
PH • the [H 3 O+] can vary a great deal!! in a neutral solution it is 1. 0 x 10 -7 M. in 1. 0 M HCI it is 1. 0 M. • a logarithmic scale (the p. H scale) is used to describe H 3 O+ concentration because it can cover a wide range of values in a concise manner. • PH stands for potency of hydrogen. • It is defined as the negative log of the H 3 O+ concentration. Ph=-log [H 3 O+] • And conversely, [H 3 O+] =10 -p. H

PH Calculations Example 1: Find the PH of 0. 0010 M HI. Example 2: Find the PH of pure water. Example 3: Find the PH of 1. 0 M HNO 3.

p. H Calculations Example 4: Find the PH of 1. 0 M Na. OH. Example 5: Find the PH of 0. 23 M HNO 3. Example 6: Find [H 3 O+] for a solution of HNO 3 with p. H=5. 2.
![p. OH • p. OH is analogous to p. H. p. OH =-log [OH-] p. OH • p. OH is analogous to p. H. p. OH =-log [OH-]](http://slidetodoc.com/presentation_image_h2/888d6c4265683ad0dcac78f335e7e2ed/image-12.jpg)
p. OH • p. OH is analogous to p. H. p. OH =-log [OH-] = 10 -p. OH
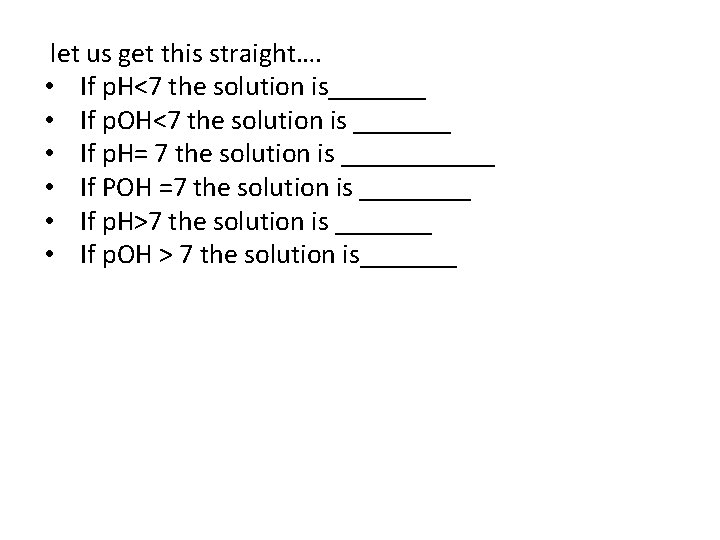
let us get this straight…. • If p. H˂7 the solution is_______ • If p. OH˂7 the solution is _______ • If p. H= 7 the solution is ______ • If POH =7 the solution is ____ • If p. H˃7 the solution is _______ • If p. OH ˃ 7 the solution is_______
![p. Kw • at any temperature: [H 3 O+][OH-]=Kw • Take the –log of p. Kw • at any temperature: [H 3 O+][OH-]=Kw • Take the –log of](http://slidetodoc.com/presentation_image_h2/888d6c4265683ad0dcac78f335e7e2ed/image-14.jpg)
p. Kw • at any temperature: [H 3 O+][OH-]=Kw • Take the –log of all terms: p. H+ p. OH= p. Kw • Specially, at 25°C: [H 3 O+][OH-] = l. 0 x l 0 -14 • And therefore: à p. H +p. OH =14
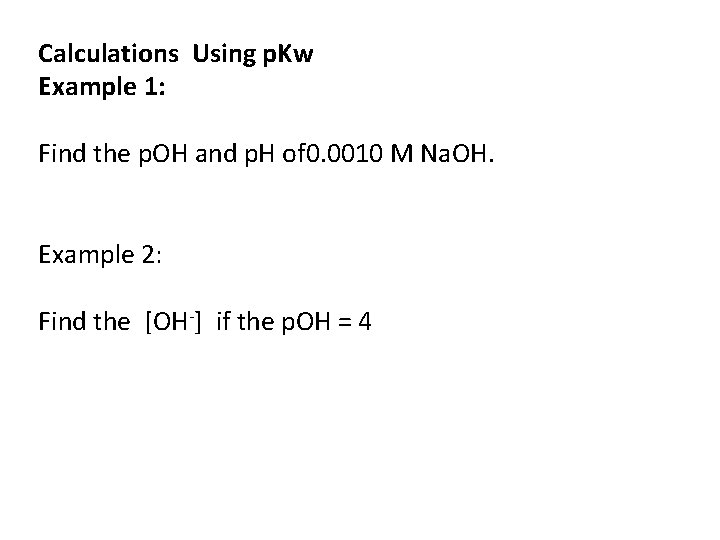
Calculations Using p. Kw Example 1: Find the p. OH and p. H of 0. 0010 M Na. OH. Example 2: Find the [OH-] if the p. OH = 4

Calculations Using p. Kw Example 3: 0. 060 g Ca(OH)2 is added to water to make 500 m. L of solution. Find the p. H i. Find [Ca(OH)2]: ii. Find the [OH-] iii. find the Po. H iv. Find p. H

Assignment PH ws 1 and 2
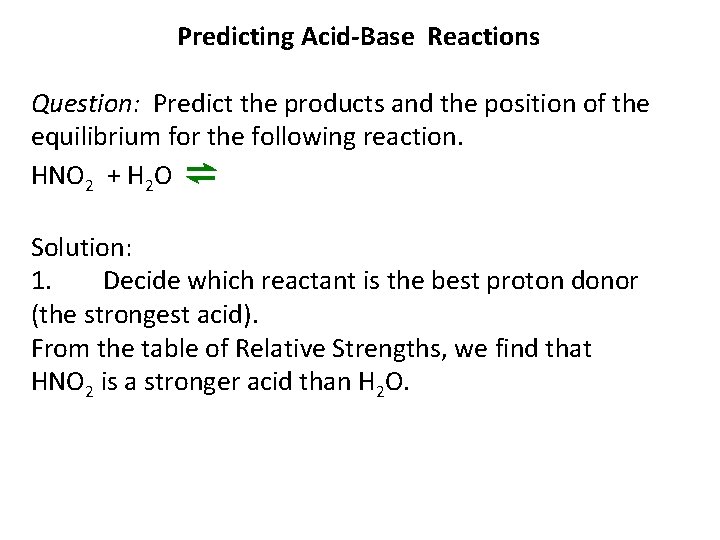
Predicting Acid-Base Reactions Question: Predict the products and the position of the equilibrium for the following reaction. HNO 2 + H 2 O ⇌ Solution: 1. Decide which reactant is the best proton donor (the strongest acid). From the table of Relative Strengths, we find that HNO 2 is a stronger acid than H 2 O.
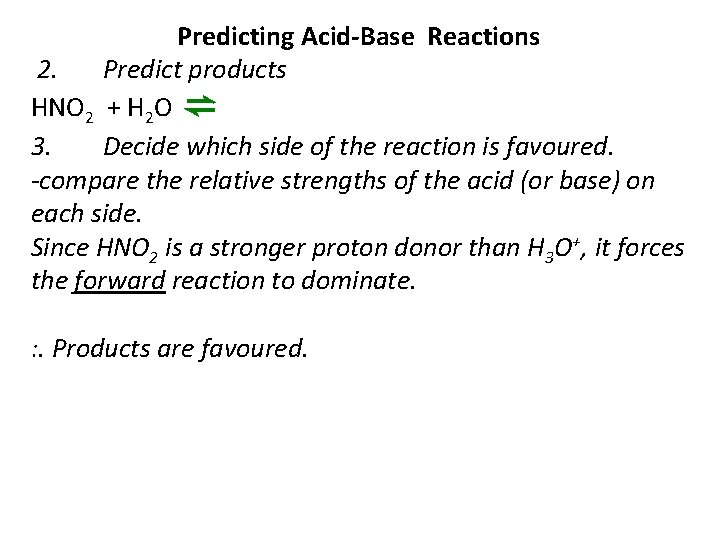
Predicting Acid-Base Reactions 2. Predict products HNO 2 + H 2 O ⇌ 3. Decide which side of the reaction is favoured. -compare the relative strengths of the acid (or base) on each side. Since HNO 2 is a stronger proton donor than H 3 O+, it forces the forward reaction to dominate. : . Products are favoured.
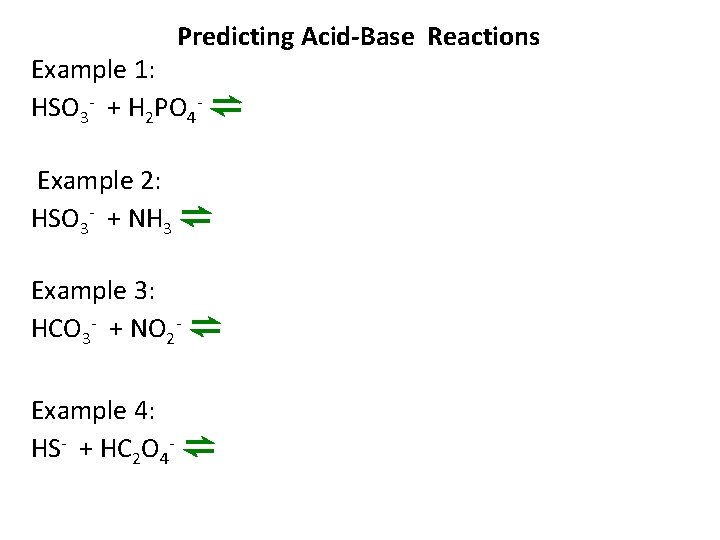
Predicting Acid-Base Reactions Example 1: HSO 3 - + H 2 PO 4 - ⇌ Example 2: HSO 3 - + NH 3 ⇌ Example 3: HCO 3 - + NO 2 - ⇌ Example 4: HS- + HC 2 O 4 - ⇌

Assignment • Acid base equilibrium ws • Hebden #38 ae, 39 ae and 40 abch, 42, 43
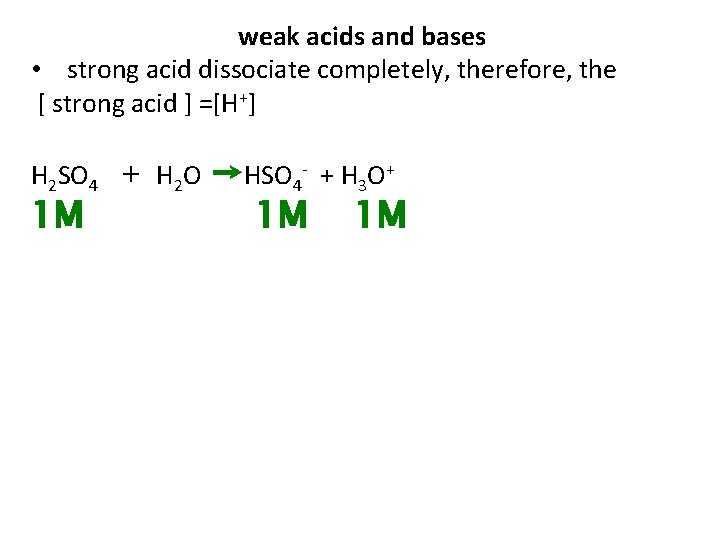
weak acids and bases • strong acid dissociate completely, therefore, the [ strong acid ] =[H+] H 2 SO 4 1 M + H 2 O →HSO 4 - + H 3 O+ 1 M 1 M
![Weak Acids do NOT dissociate completely. The [weak acid] ≠ [H+] (is not equal Weak Acids do NOT dissociate completely. The [weak acid] ≠ [H+] (is not equal](http://slidetodoc.com/presentation_image_h2/888d6c4265683ad0dcac78f335e7e2ed/image-23.jpg)
Weak Acids do NOT dissociate completely. The [weak acid] ≠ [H+] (is not equal to) HF + H 2 O ⇌ F - + H 3 O+ 1 M ˂1 M
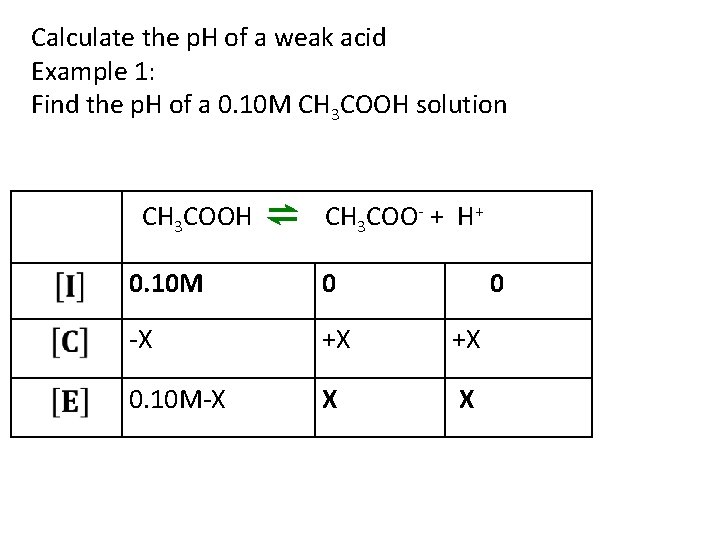
Calculate the p. H of a weak acid Example 1: Find the p. H of a 0. 10 M CH 3 COOH solution CH 3 COOH ⇌ CH 3 COO- + H+ 0. 10 M 0 0 -X +X +X 0. 10 M-X X X
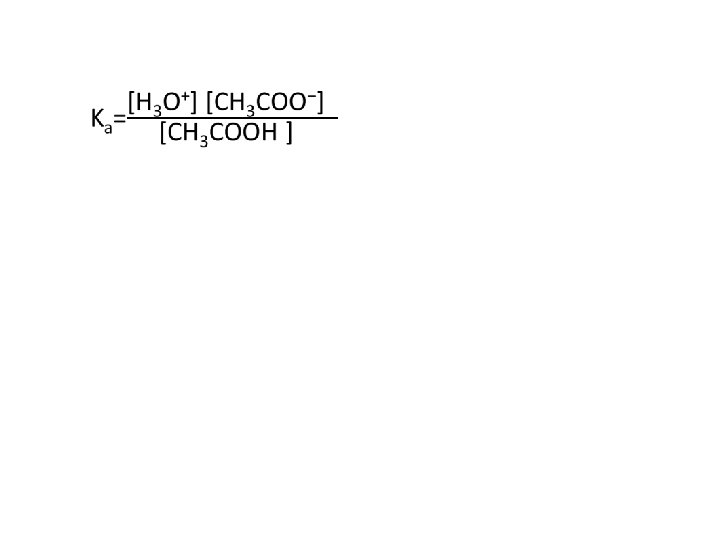
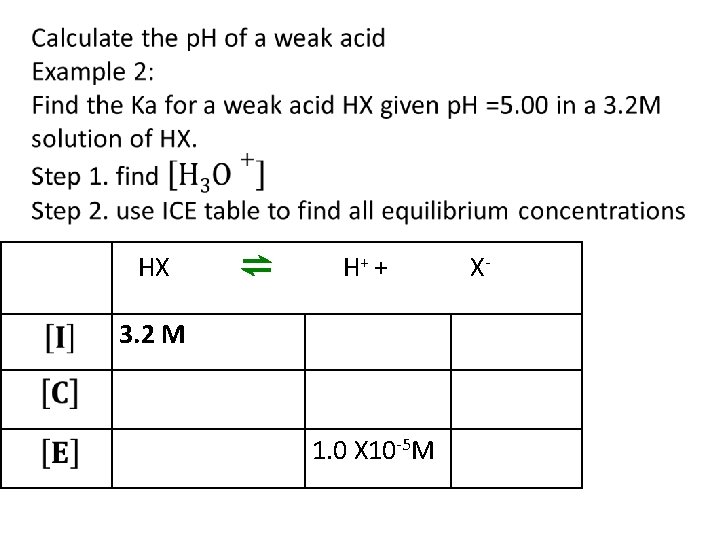
HX ⇌ H+ + 3. 2 M 1. 0 X 10 -5 M X-


Assignment • Hebden 74 -76, 80, 82
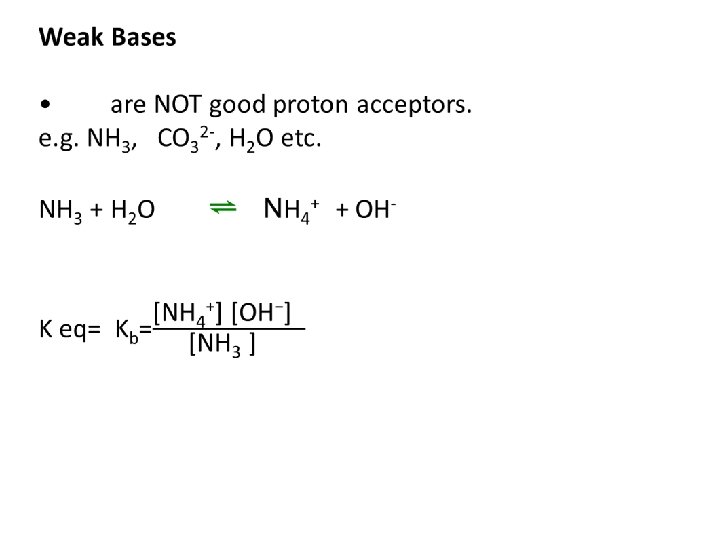
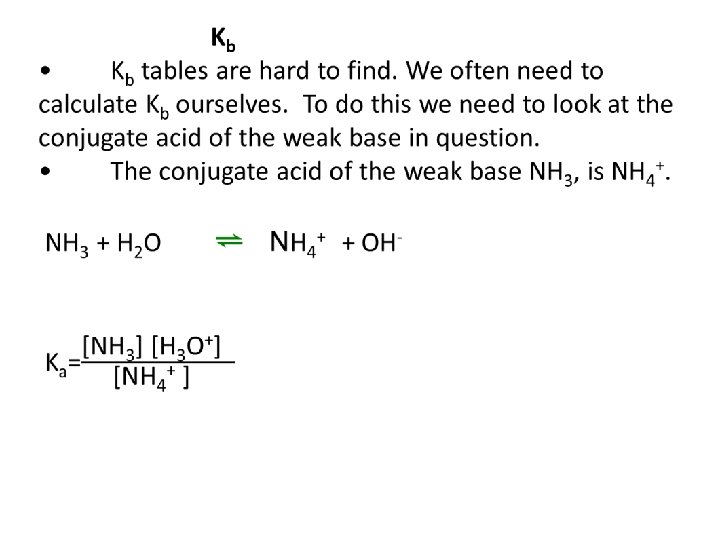
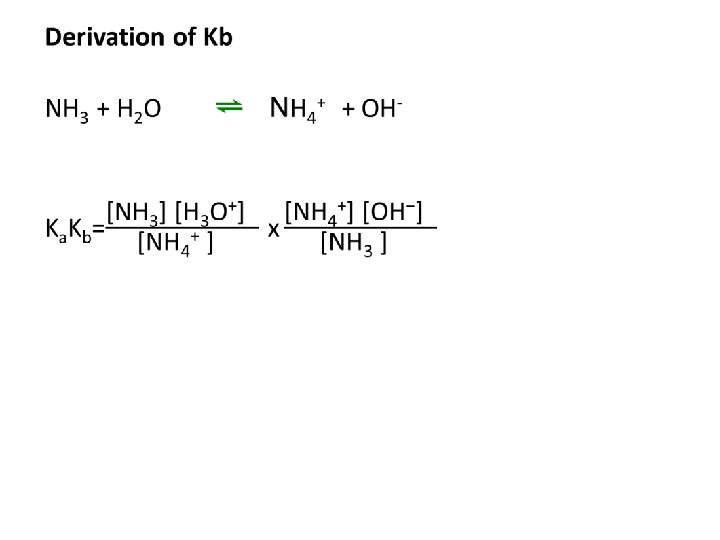
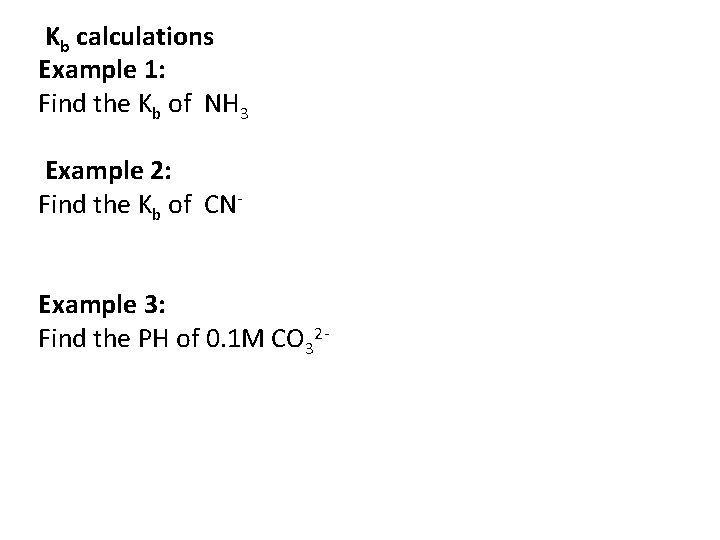
Kb calculations Example 1: Find the Kb of NH 3 Example 2: Find the Kb of CNExample 3: Find the PH of 0. 1 M CO 32 -

Assignment • Hebden #85, 87, 89 • WORKBOOK

UNIT review Assignment • Acid base review sheet • Acid base quiz • Unit D, E test
- Slides: 35