Unit Conversions R A Hill Unit Conversions Within
























































- Slides: 88

Unit Conversions © R. A. Hill

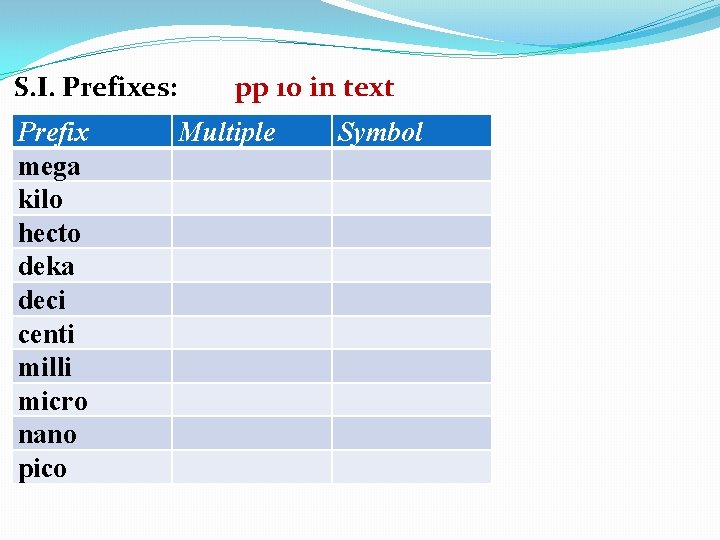
Unit Conversions Within the S. I. System Need to know prefixes used in S. I. system

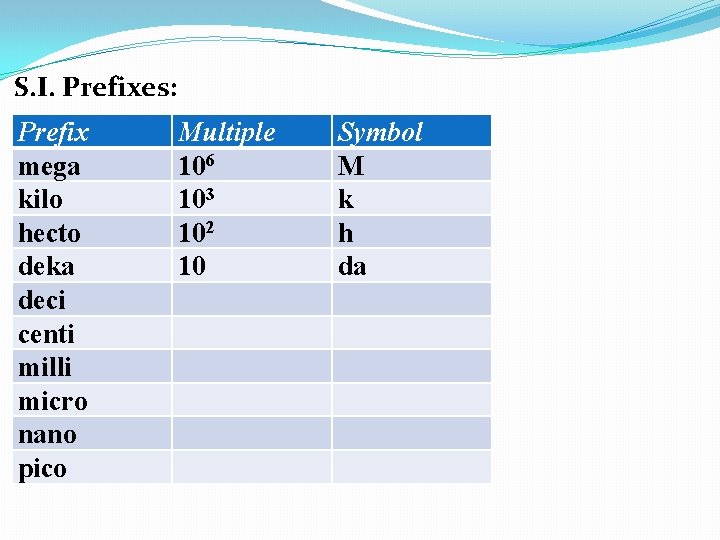
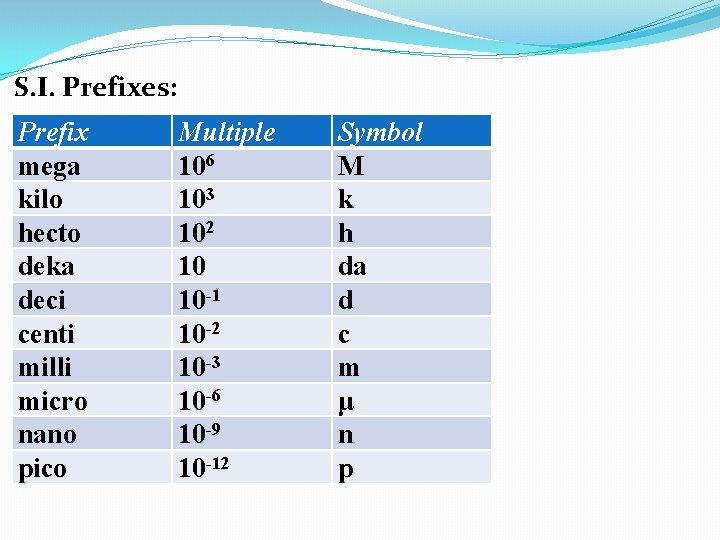
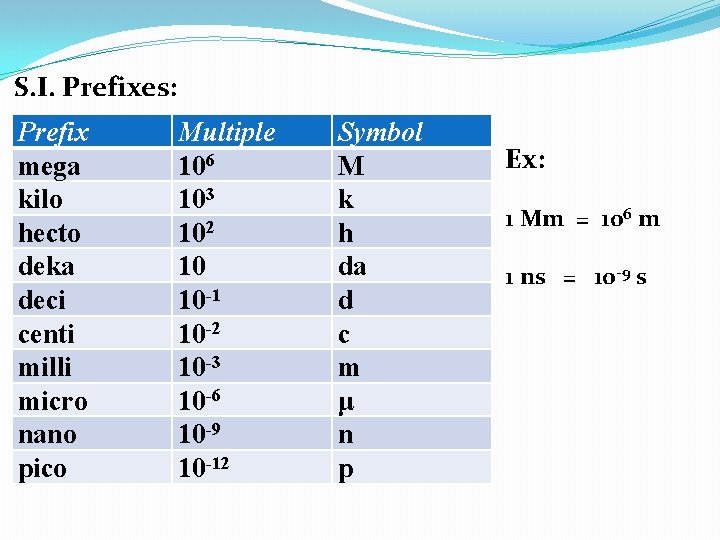
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico pp 10 in text Multiple Symbol

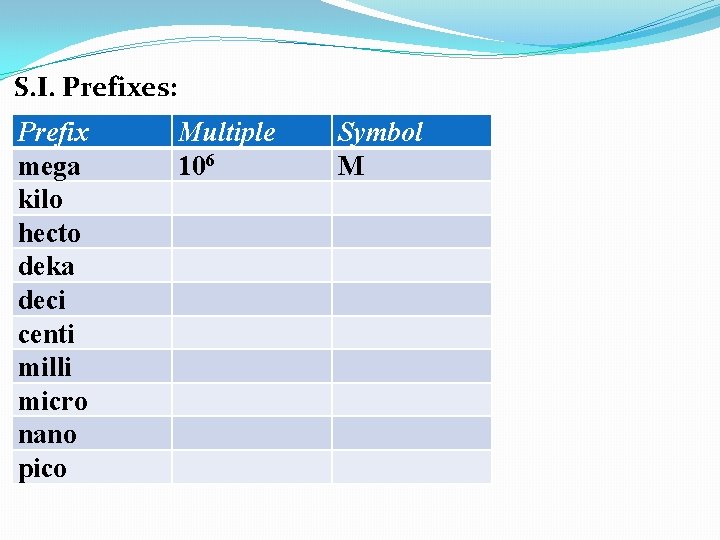
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 Symbol M

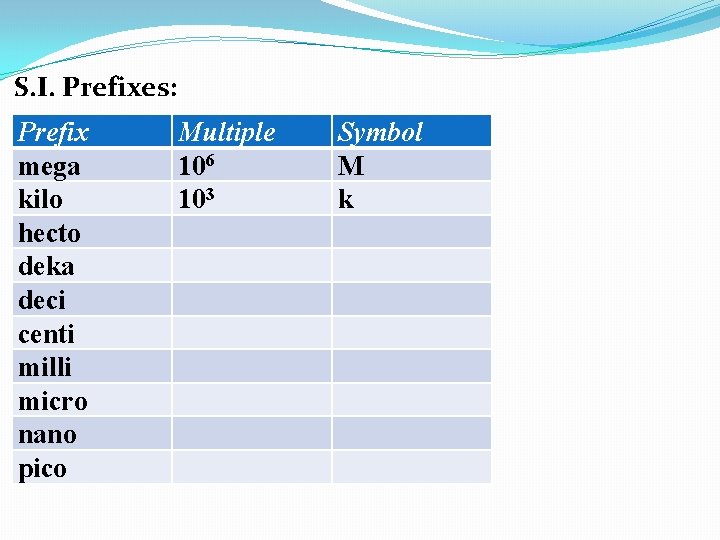
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 Symbol M k

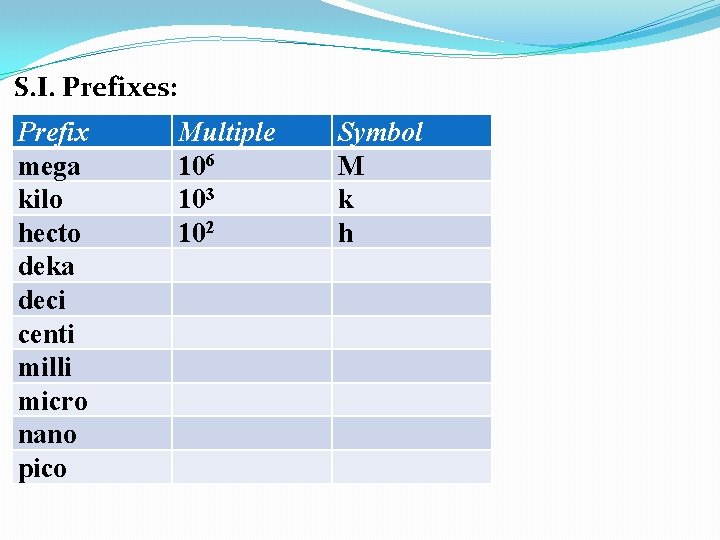
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 Symbol M k h

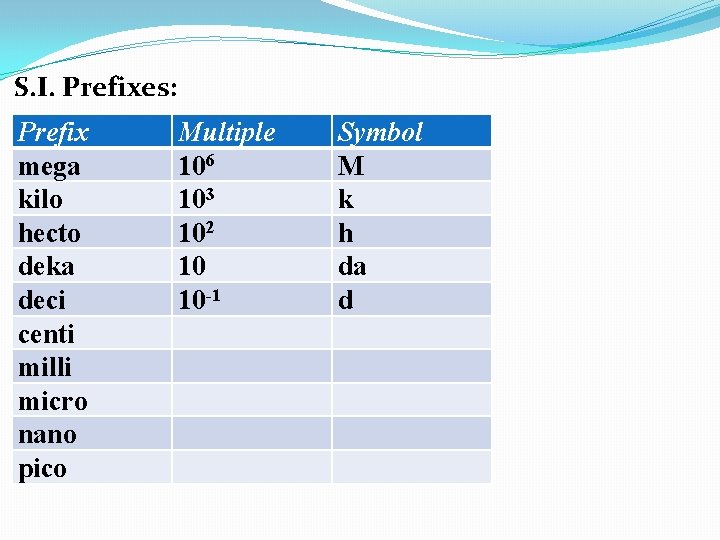
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 Symbol M k h da

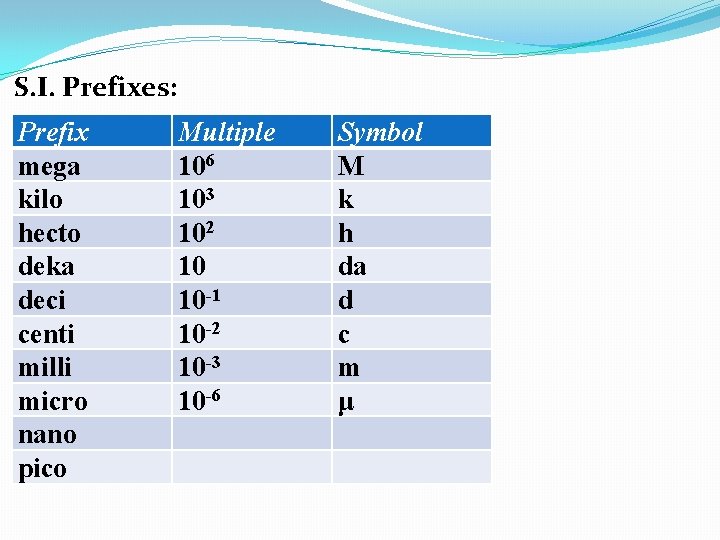
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 Symbol M k h da d

S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 Symbol M k h da d c

S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 Symbol M k h da d c m

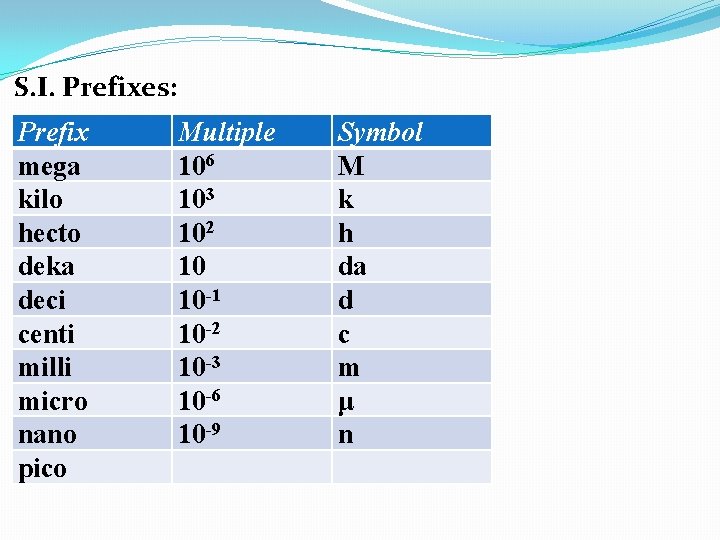
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 Symbol M k h da d c m μ

S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 Symbol M k h da d c m μ n

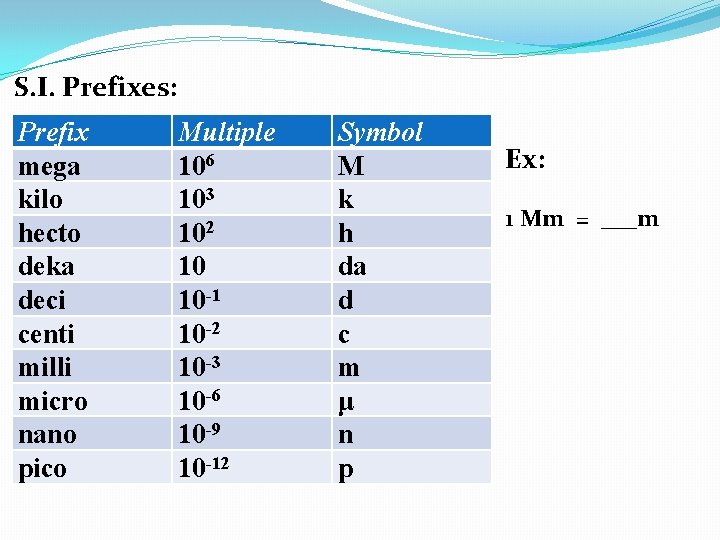
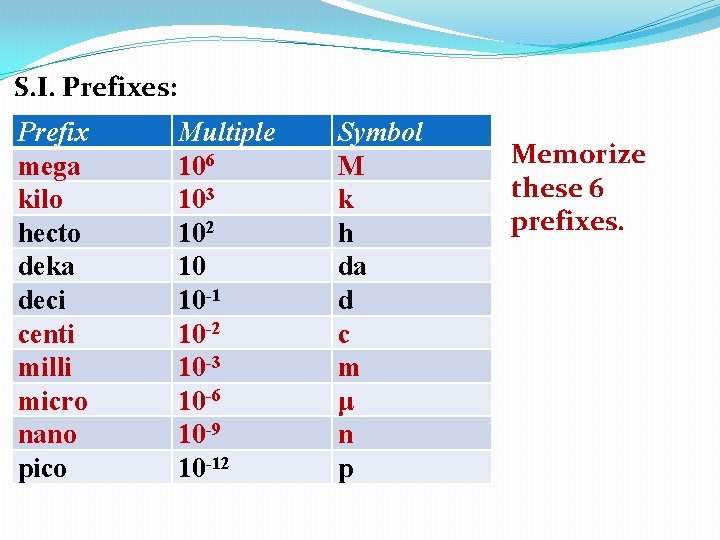
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p

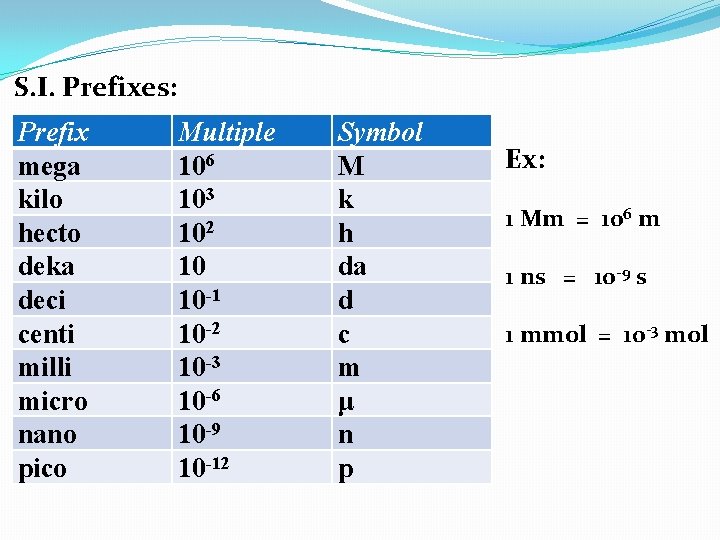
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p Ex: 1 Mm = ___m

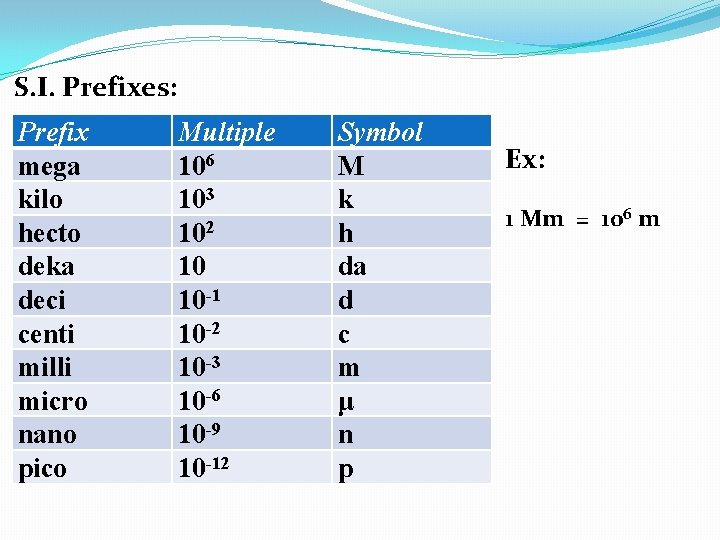
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p Ex: 1 Mm = 106 m

S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p Ex: 1 Mm = 106 m 1 ns = ____s

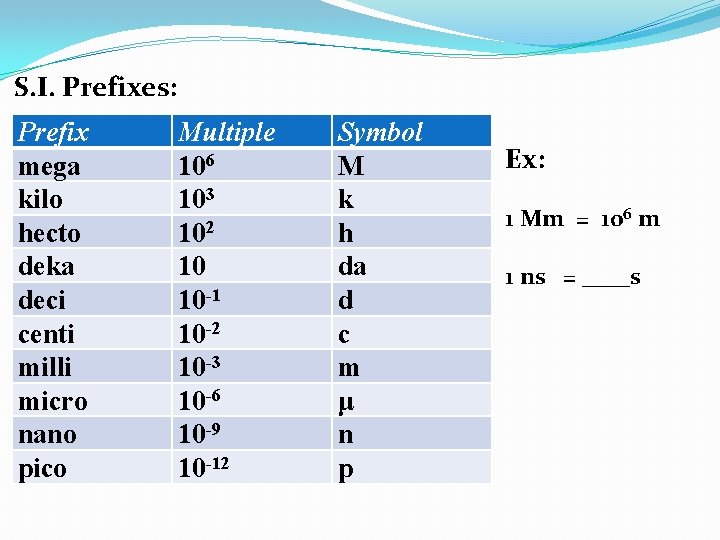
S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p Ex: 1 Mm = 106 m 1 ns = 10 -9 s

S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p Ex: 1 Mm = 106 m 1 ns = 10 -9 s 1 mmol = ___mol

S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p Ex: 1 Mm = 106 m 1 ns = 10 -9 s 1 mmol = 10 -3 mol

S. I. Prefixes: Prefix mega kilo hecto deka deci centi milli micro nano pico Multiple 106 103 102 10 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Symbol M k h da d c m μ n p Memorize these 6 prefixes.

S. F. in Nonmeasured Numbers If a number is not measured then it does not have an estimated digit in it. treat it as if it has ∞ S. F. Ex: #s in definitions 12 objects = 1 dozen objects 1 mm = 10− 3 m counting number of objects i. e. 15 people

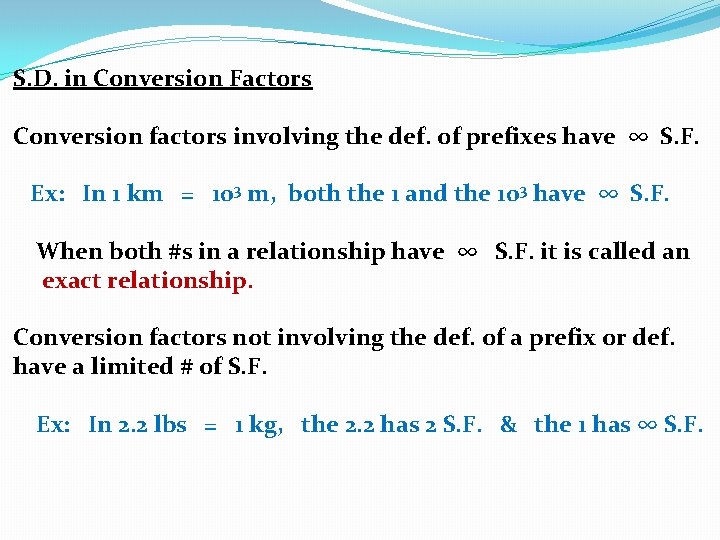
S. D. in Conversion Factors Conversion factors involving the def. of prefixes have ∞ S. F. Ex: In 1 km = 103 m, both the 1 and the 103 have ∞ S. F. When both #s in a relationship have ∞ S. F. it is called an exact relationship. Conversion factors not involving the def. of a prefix or def. have a limited # of S. F. Ex: In 2. 2 lbs = 1 kg, the 2. 2 has 2 S. F. & the 1 has ∞ S. F.

Unit Conversions Within the S. I. System 243 mm = ? m

Unit Conversions Within the S. I. System 0. 01 km = ? cm

Unit Conversions Between Different Systems of Measurement 3. 21 cal = ? J

Unit Conversions Between Different Systems of Measurement 8. 9 lbs = ? g

Unit Conversions Between Different Systems of Measurement 0. 20 km = ? in

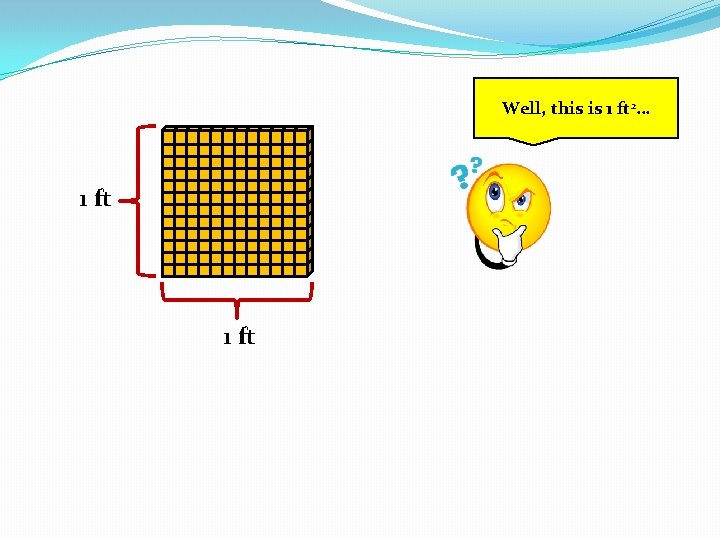
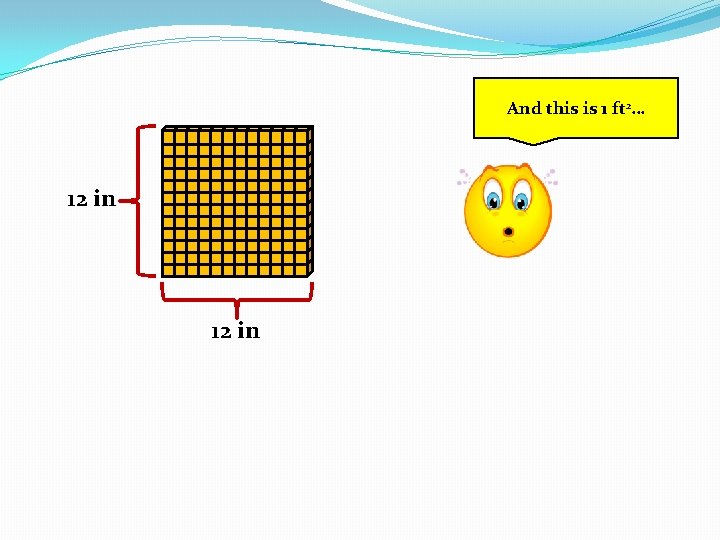
Squared and Cubed Conversion Factors 562 in 2 = ? ft 2 562 in 2 1 ft 2 12 in 2 = Wait… 1 ft = 12 in but does 1 ft 2 = 12 in 2 ?

Well, this is 1 ft 2… 1 ft

And this is 1 ft 2… 12 in

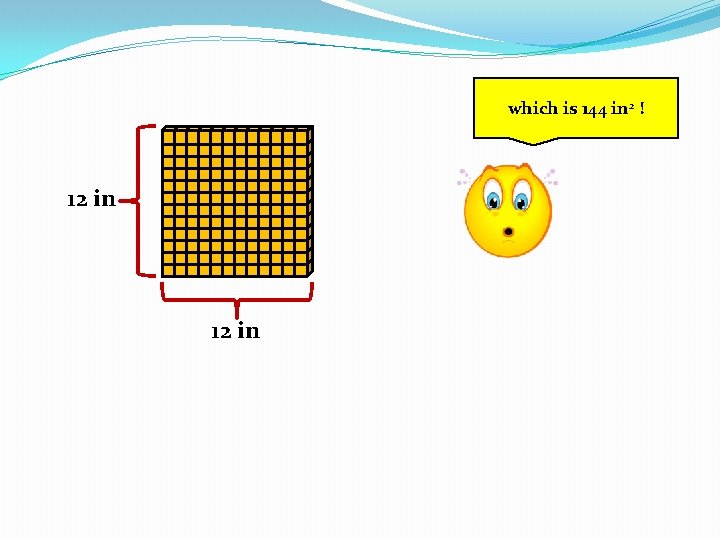
which is 144 in 2 ! 12 in

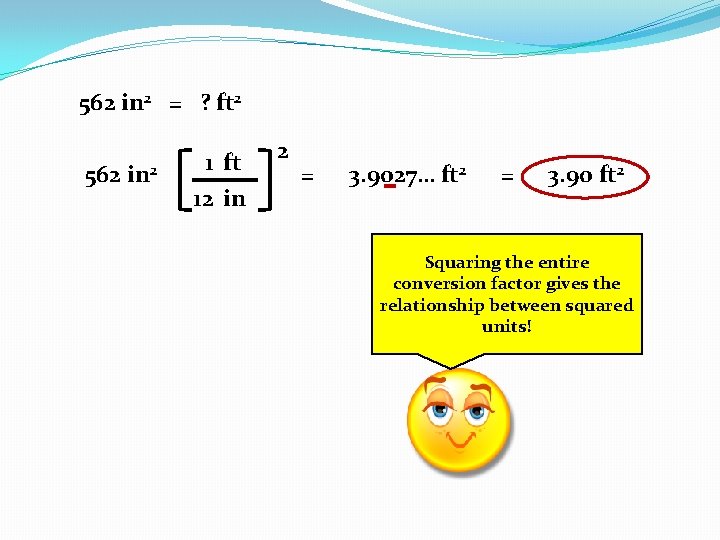
562 in 2 = ? ft 2 562 in 2 1 ft 12 in 2 = 3. 9027… ft 2 = 3. 90 ft 2 Squaring the entire conversion Lets tryfactor this gives the relationship again… between squared units!

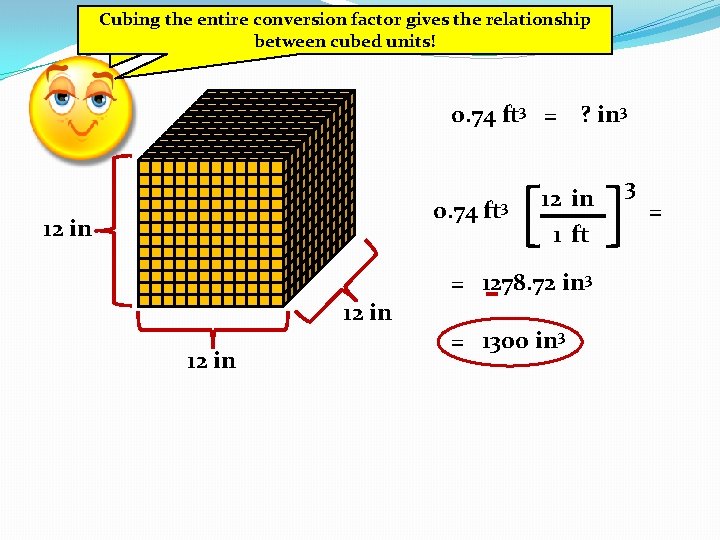
Cubing entire 3 and So for ftthe in 3 …conversion factor gives the relationship between cubed units! 0. 74 ft 3 = ? in 3 0. 74 ft 3 12 in 1 ft = 1278. 72 in 3 12 in = 1300 in 3 3 =

Unit Conversions Between Different Systems of Measurement 3. 25 g/ml = ? lbs/ft 3

Unit Conversions with Equivalent Measurements density (def) – the mass per volume for a substance. What is the mass of 1. 25 l of Hg (density = 13. 6 g/ml)?


atomic weights (def)

atomic weights (def) – the average mass of an atom of an element relative to an atom of carbon-12 being assigned a mass of exactly 12 a. m. u.

atomic weights (def) – the average mass of an atom of an element relative to an atom of carbon-12 being assigned a mass of exactly 12 a. m. u.

atomic weights (def) – the average mass of an atom of an element relative to an atom of carbon-12 being assigned a mass of exactly 12 a. m. u. = atomic mass unit

atomic weights (def) – the average mass of an atom of an element relative to an atom of carbon-12 being assigned a mass of exactly 12 a. m. u. = atomic mass unit These can be found on the periodic chart. Ex: hydrogen

atomic weights (def) – the average mass of an atom of an element relative to an atom of carbon-12 being assigned a mass of exactly 12 a. m. u. = atomic mass unit These can be found on the periodic chart. Ex: hydrogen 1. 008

formula weight

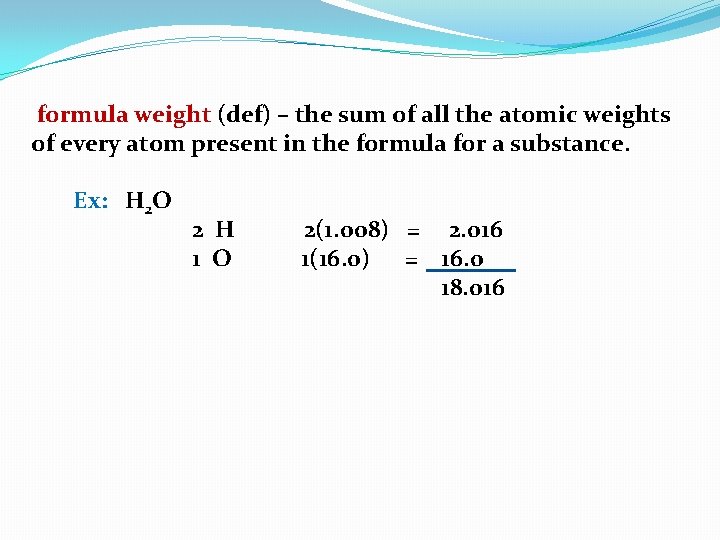
formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance.

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O 2 H 1 O

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O 2 H 1 O (1. 008)

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O 2 H 1 O 2(1. 008)

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O 2 H 1 O 2(1. 008) (16. 0)

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O 2 H 1 O 2(1. 008) 1(16. 0)

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O 2 H 1 O 2(1. 008) = 2. 016 1(16. 0) = 16. 0 18. 016

formula weight (def) – the sum of all the atomic weights of every atom present in the formula for a substance. Ex: H 2 O 2 H 1 O 2(1. 008) = 2. 016 1(16. 0) = 16. 0 18. 016 = 18. 0 = f. w.

mole (def)

mole (def) – a quantity of substance that contains as many particles as there atoms present in exactly 12 g of carbon-12.

mole (def) – a quantity of substance that contains as many particles as there atoms present in exactly 12 g of carbon-12. This # has been experimentally determined to be:

mole (def) – a quantity of substance that contains as many particles as there atoms present in exactly 12 g of carbon-12. This # has been experimentally determined to be: 6. 022 x 1023

mole (def) – a quantity of substance that contains as many particles as there atoms present in exactly 12 g of carbon-12. This # has been experimentally determined to be: 6. 022 x 1023 Avogadro’s Number

mole (def) – a quantity of substance that contains as many particles as there atoms present in exactly 12 g of carbon-12. This # has been experimentally determined to be: 6. 022 x 1023 Avogadro’s Number In problems, the word “particles” is replaced with the term “atom”, “molecule” or whatever is appropriate.

molar mass

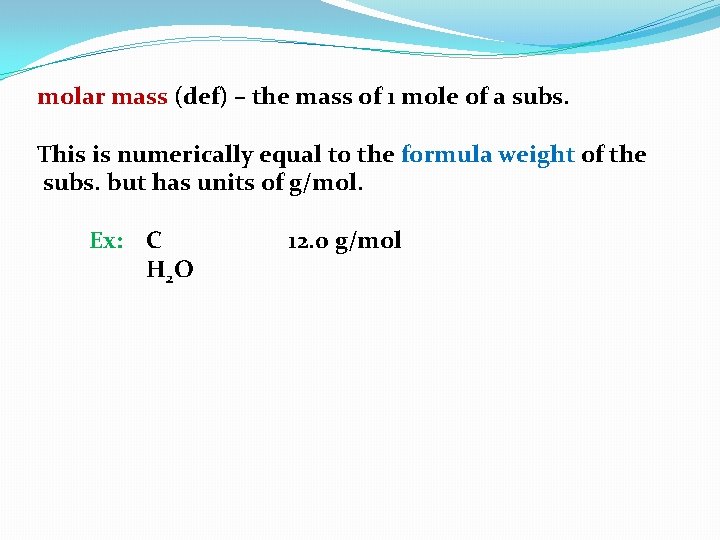
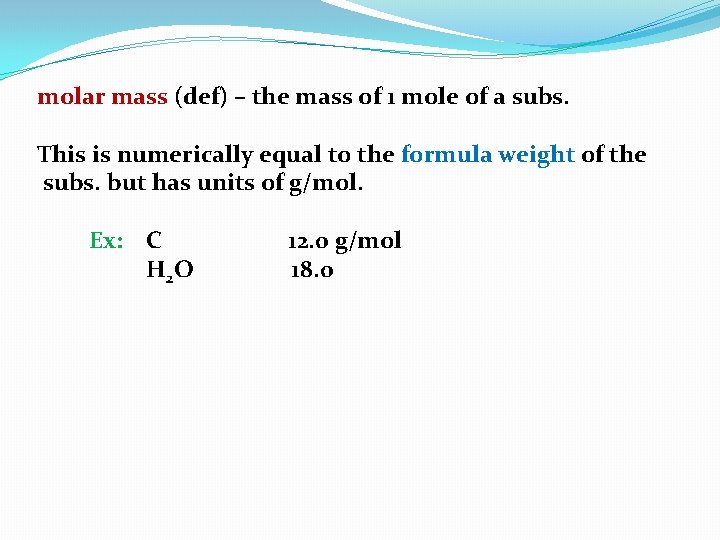
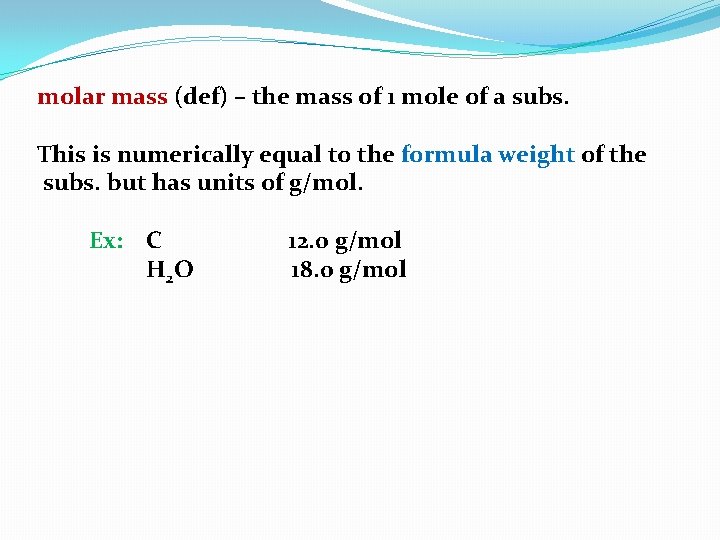
molar mass (def) – the mass of 1 mole of a subs.

molar mass (def) – the mass of 1 mole of a subs. This is numerically equal to the formula weight of the subs. but has units of g/mol.

molar mass (def) – the mass of 1 mole of a subs. This is numerically equal to the formula weight of the subs. but has units of g/mol. Ex: C

molar mass (def) – the mass of 1 mole of a subs. This is numerically equal to the formula weight of the subs. but has units of g/mol. Ex: C 12. 0

molar mass (def) – the mass of 1 mole of a subs. This is numerically equal to the formula weight of the subs. but has units of g/mol. Ex: C 12. 0 g/mol

molar mass (def) – the mass of 1 mole of a subs. This is numerically equal to the formula weight of the subs. but has units of g/mol. Ex: C H 2 O 12. 0 g/mol

molar mass (def) – the mass of 1 mole of a subs. This is numerically equal to the formula weight of the subs. but has units of g/mol. Ex: C H 2 O 12. 0 g/mol 18. 0

molar mass (def) – the mass of 1 mole of a subs. This is numerically equal to the formula weight of the subs. but has units of g/mol. Ex: C H 2 O 12. 0 g/mol 18. 0 g/mol

molar volume

molar volume (def) – the volume of 1 mole of a subs.

molar volume (def) – the volume of 1 mole of a subs. The molar volume of any gas at S. T. P. is

molar volume (def) – the volume of 1 mole of a subs. The molar volume of any gas at S. T. P. is S. T. P. = standard temperature & pressure =

molar volume (def) – the volume of 1 mole of a subs. The molar volume of any gas at S. T. P. is S. T. P. = standard temperature & pressure = Oo. C & 1 atm

molar volume (def) – the volume of 1 mole of a subs. The molar volume of any gas at S. T. P. is 22. 4 l/mol. S. T. P. = standard temperature & pressure = Oo. C & 1 atm

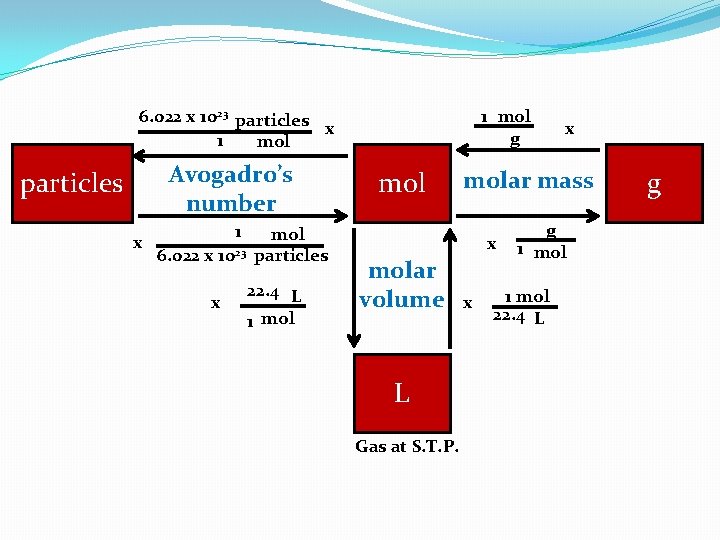
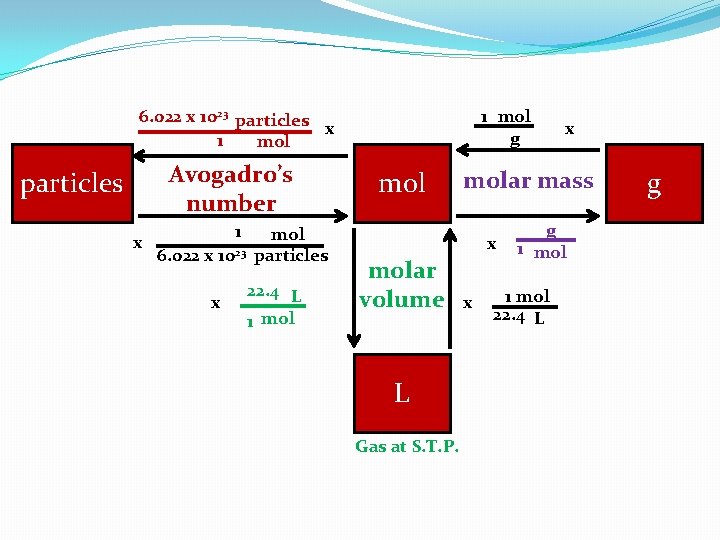
6. 022 x 1023 particles x 1 mol Avogadro’s number particles x 1 6. 022 x 1023 x mol particles 22. 4 L 1 mol g molar mass x molar volume L Gas at S. T. P. x x g 1 mol 22. 4 L g

6. 022 x 1023 particles x 1 mol Avogadro’s number particles x 1 6. 022 x 1023 x mol particles 22. 4 L 1 mol g molar mass x molar volume L Gas at S. T. P. x x g 1 mol 22. 4 L g

0. 25 mol H 2 O contains how many H atoms?

What is the mass of 24. 7 mmol of Fe 2(SO 4)3?

0. 458 mol of CH 4 gas occupies how many ml at S. T. P. ?

A 1 carat diamond (pure C) has a mass of 0. 200 g. How many C atoms are present in a 1 carat diamond?

Octane (C 8 H 18) has a density of 0. 80 g/ml. What is the volume in ml of 1. 00 x 1024 molecules of C 8 H 18?

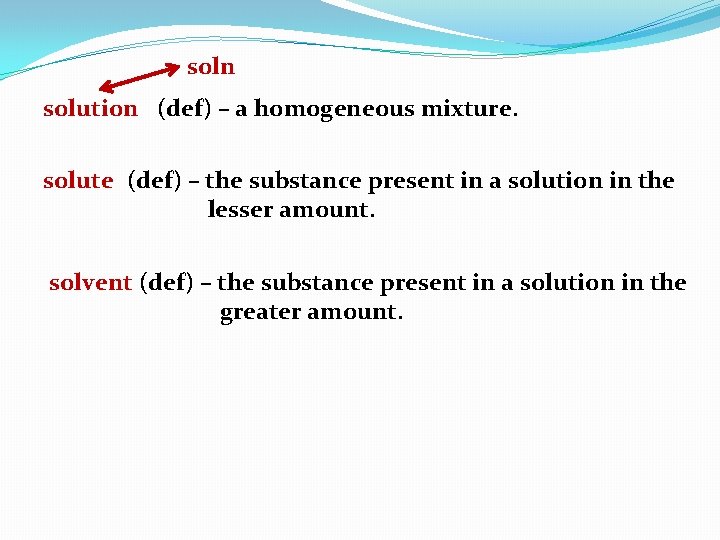
soln solution (def) – a homogeneous mixture. solute (def) – the substance present in a solution in the lesser amount. solvent (def) – the substance present in a solution in the greater amount.

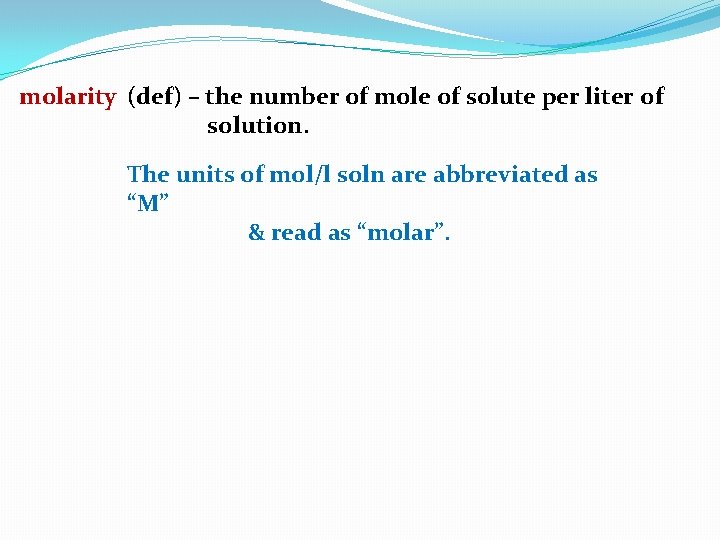
molarity (def) – the number of mole of solute per liter of solution. The units of mol/l soln are abbreviated as “M” & read as “molar”.

Ex: volumetric flask 1 liter mark Add H 2 O until V of soln = 1 liter 3 mol Na. Cl 3 mol of Na. Cl 1 l Na. Cl soln 3 M Na. Cl soln Note: (1) less than 1 liter of solvent is added & the amount will differ with each solute (2) in making solns, volumes are not always additive ex: 50 ml H 2 O + 50 ml C 3 H 7 OH = 96 ml soln …V of H 2 O + V of C 3 H 7 OH > V of soln V of NH 4 Cl + V of H 2 O < V of soln

How would 1. 000 l of 2. 00 M Ca. Cl 2 be prepared?

1. 52 g of Na. OH is diluted with H 2 O until a solution with a total volume of 250. 0 ml is obtained. What is the molarity of the Na. OH solution?

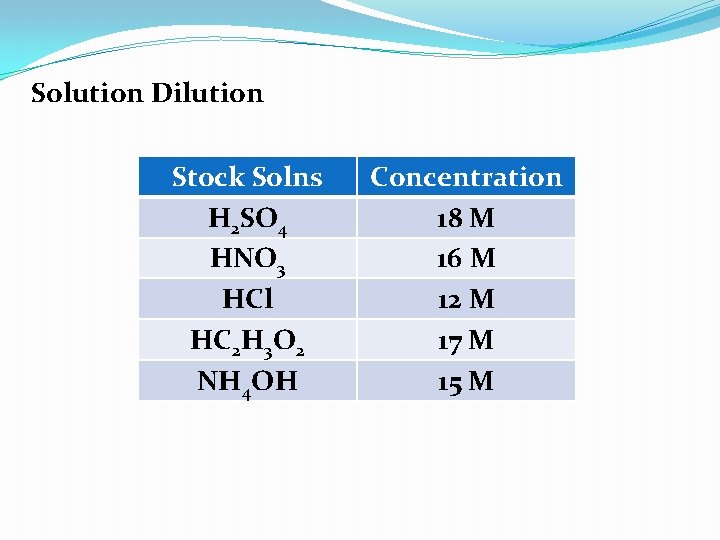
Solution Dilution Stock Solns H 2 SO 4 HNO 3 HCl HC 2 H 3 O 2 NH 4 OH Concentration 18 M 16 M 12 M 17 M 15 M

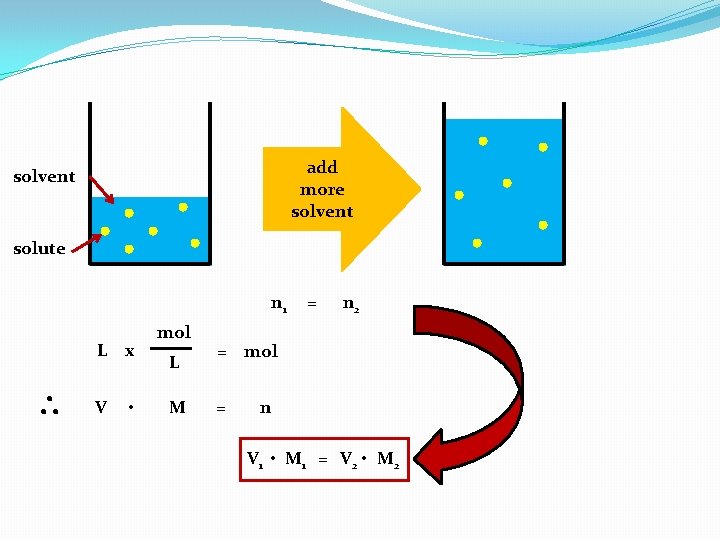
add more solvent solute n 1 L x V • mol L M = n 2 = mol = n V 1 • M 1 = V 2 • M 2

How would 100. 0 ml of 1 M HNO 3 be prepared?