Unit Cell of Crystal Structure AL Chemistry Definition


- Slides: 20

Unit Cell of Crystal Structure AL Chemistry # Definition of “Unit Cell”: A unit cell is the smallest basic portion of the crystal lattice that, repeatedly stacked together in three dimensions , can generate the entire crystal structure. [2003 Paper I, Q. 4(b)] p. 1

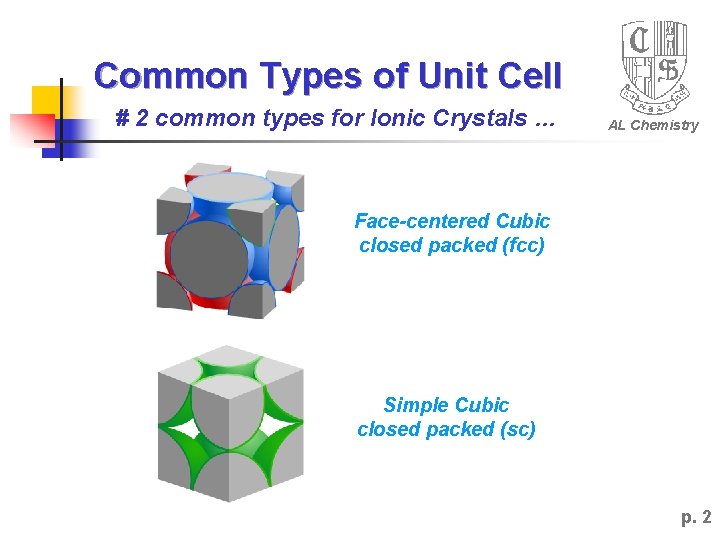
Common Types of Unit Cell # 2 common types for Ionic Crystals … AL Chemistry Face-centered Cubic closed packed (fcc) Simple Cubic closed packed (sc) p. 2

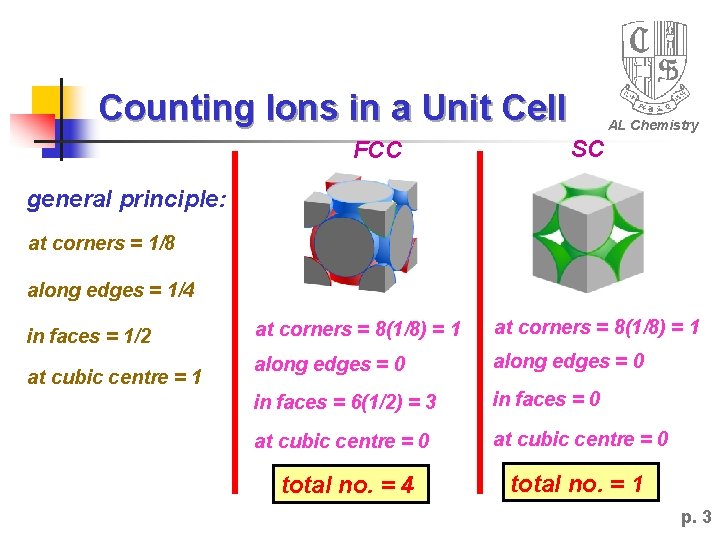
Counting Ions in a Unit Cell FCC AL Chemistry SC general principle: at corners = 1/8 along edges = 1/4 in faces = 1/2 at cubic centre = 1 at corners = 8(1/8) = 1 along edges = 0 in faces = 6(1/2) = 3 in faces = 0 at cubic centre = 0 total no. = 4 total no. = 1 p. 3

Generating of entire Lattice AL Chemistry p. 4

Ionic Crystals AL Chemistry the 3 -dimensional arrangement of ions. ** General Bonding considerations The bonding forces should be maximized by packing as many cations around each anion, and as many cations around each anion as is possible. but it depends on the relative size of cation and anion. p. 5

How do the anion and cation pack together? AL Chemistry To visualize the structures in terms of a closed packed arrangement of the larger anions (FCC or SC), with the cations occupying the vacant sites between the close packed layers. The number of nearest neighbor ions of opposite charge is called the coordination number. p. 6

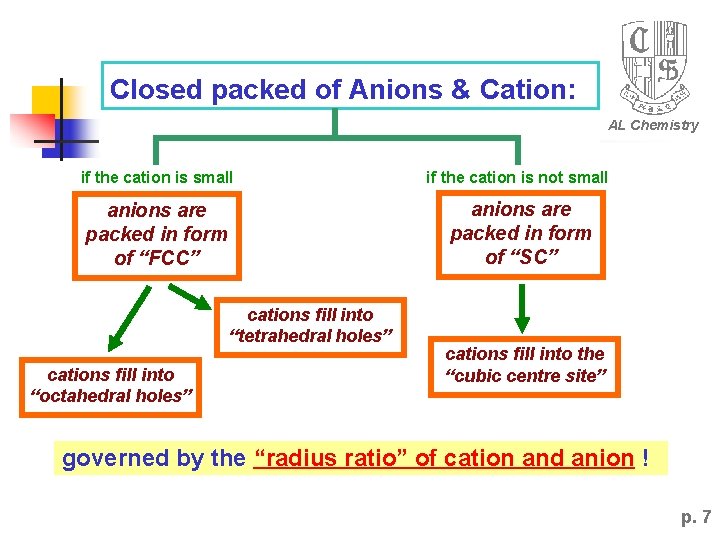
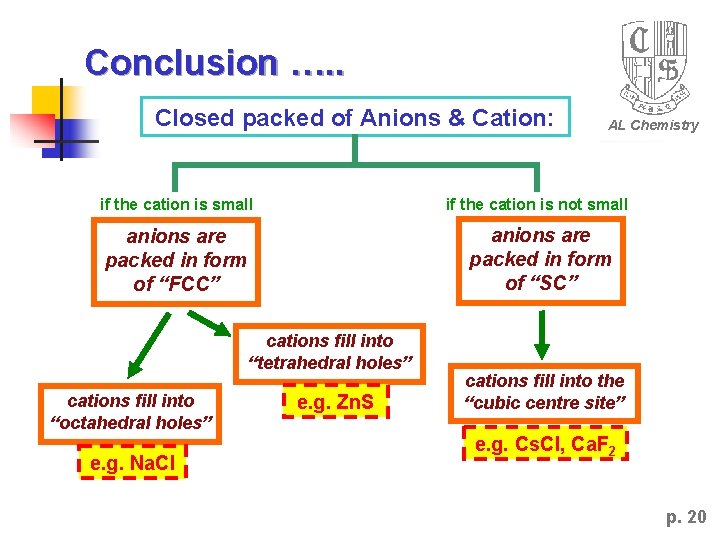
Closed packed of Anions & Cation: AL Chemistry if the cation is small if the cation is not small anions are packed in form of “FCC” anions are packed in form of “SC” cations fill into “tetrahedral holes” cations fill into “octahedral holes” cations fill into the “cubic centre site” governed by the “radius ratio” of cation and anion ! p. 7

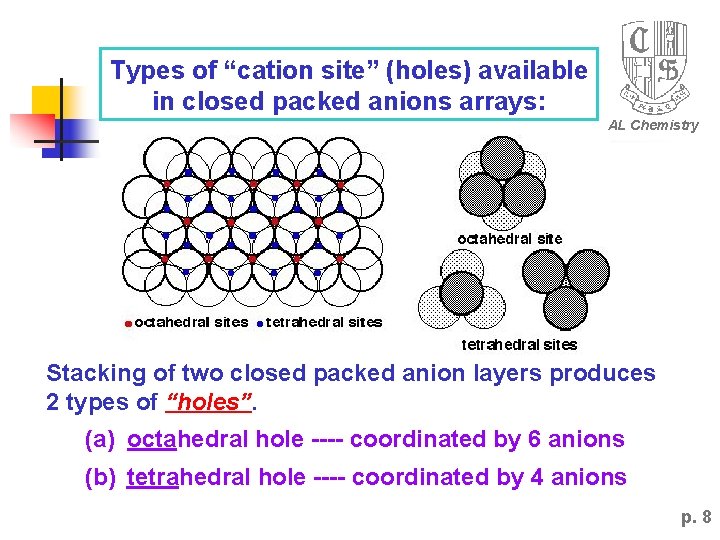
Types of “cation site” (holes) available in closed packed anions arrays: AL Chemistry Stacking of two closed packed anion layers produces 2 types of “holes”. (a) octahedral hole ---- coordinated by 6 anions (b) tetrahedral hole ---- coordinated by 4 anions p. 8

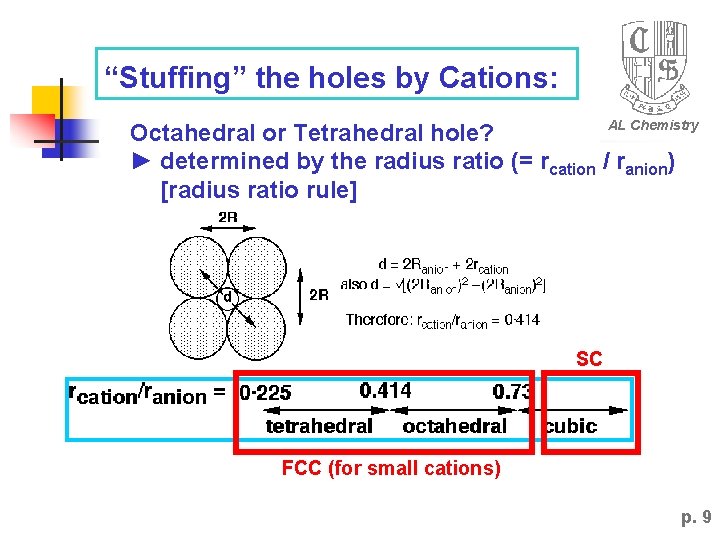
“Stuffing” the holes by Cations: AL Chemistry Octahedral or Tetrahedral hole? ► determined by the radius ratio (= rcation / ranion) [radius ratio rule] SC FCC (for small cations) p. 9

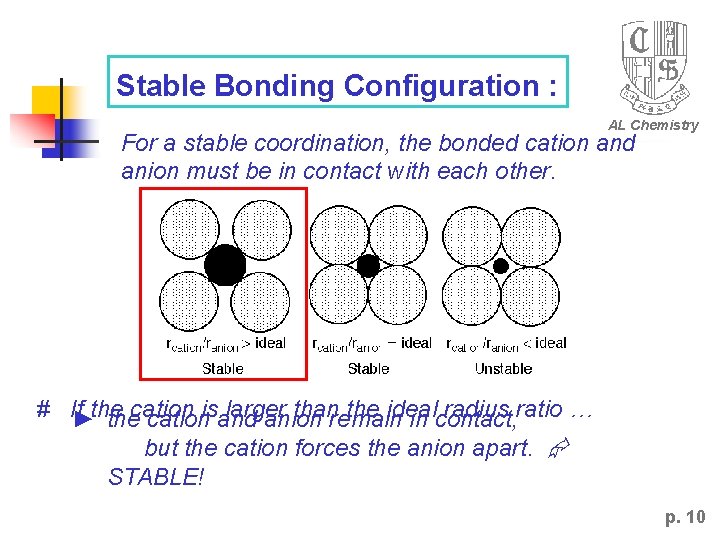
Stable Bonding Configuration : AL Chemistry For a stable coordination, the bonded cation and anion must be in contact with each other. # If►the larger thanremain the ideal radius ratio … thecationisand anion in contact, but the cation forces the anion apart. STABLE! p. 10

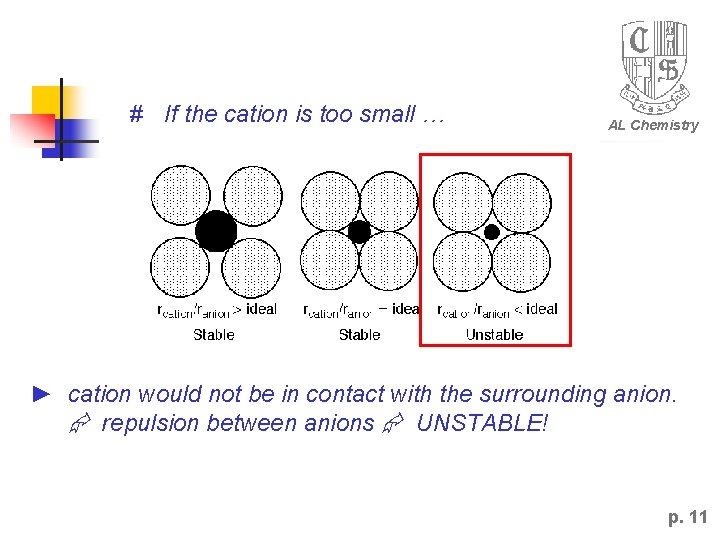
# If the cation is too small … AL Chemistry ► cation would not be in contact with the surrounding anion. repulsion between anions UNSTABLE! p. 11

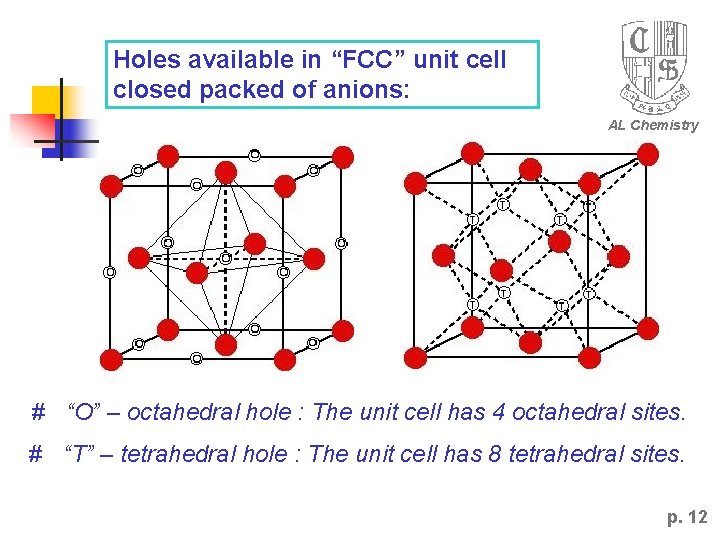
Holes available in “FCC” unit cell closed packed of anions: AL Chemistry # “O” – octahedral hole : The unit cell has 4 octahedral sites. # “T” – tetrahedral hole : The unit cell has 8 tetrahedral sites. p. 12

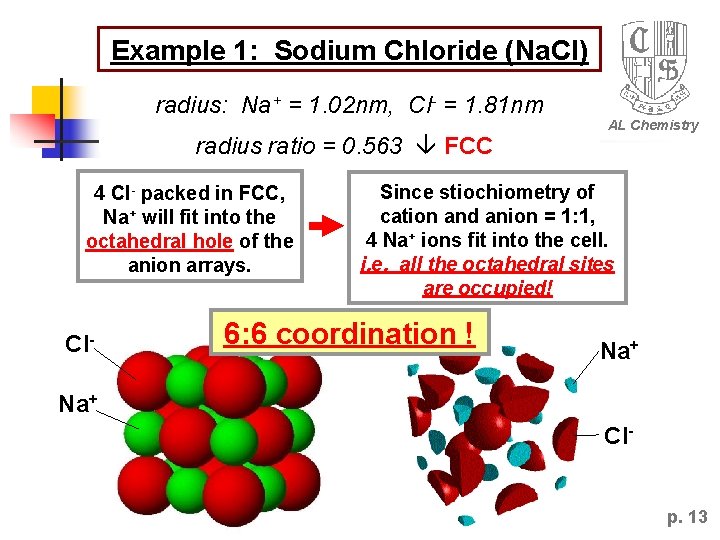
Example 1: Sodium Chloride (Na. Cl) radius: Na+ = 1. 02 nm, Cl- = 1. 81 nm radius ratio = 0. 563 FCC 4 Cl- packed in FCC, Na+ will fit into the octahedral hole of the anion arrays. Cl- AL Chemistry Since stiochiometry of cation and anion = 1: 1, 4 Na+ ions fit into the cell. i. e. all the octahedral sites are occupied! 6: 6 coordination ! Na+ Clp. 13

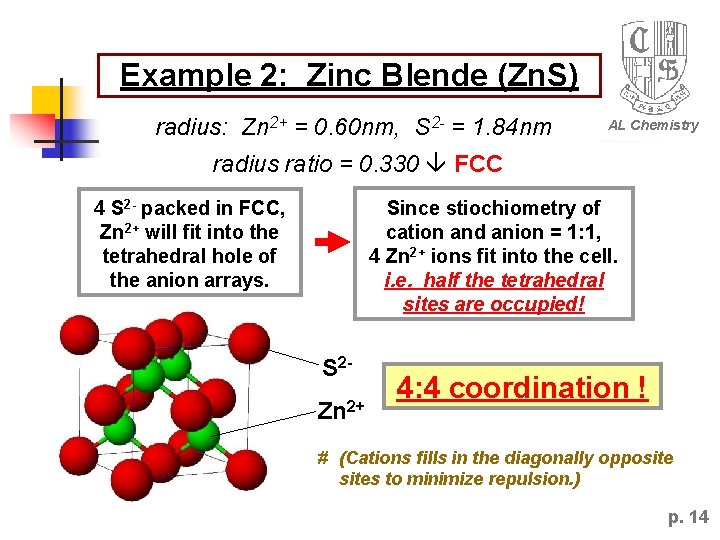
Example 2: Zinc Blende (Zn. S) radius: Zn 2+ = 0. 60 nm, S 2 - = 1. 84 nm AL Chemistry radius ratio = 0. 330 FCC Since stiochiometry of cation and anion = 1: 1, 4 Zn 2+ ions fit into the cell. i. e. half the tetrahedral sites are occupied! 4 S 2 - packed in FCC, Zn 2+ will fit into the tetrahedral hole of the anion arrays. S 2 Zn 2+ 4: 4 coordination ! # (Cations fills in the diagonally opposites to minimize repulsion. ) p. 14

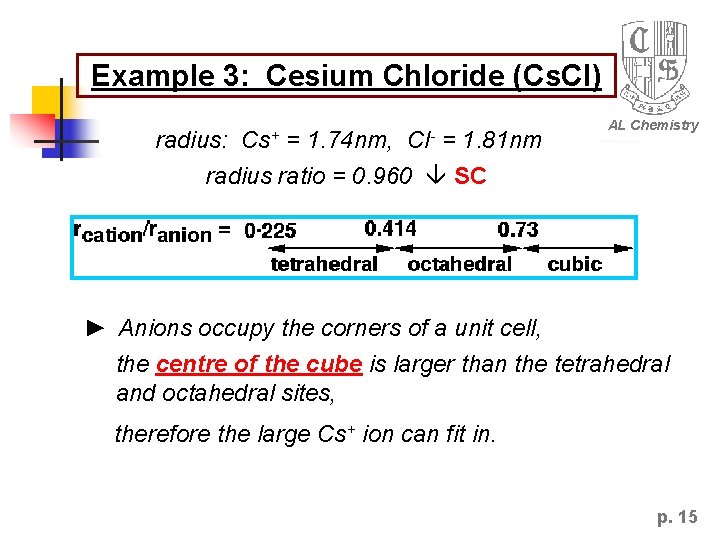
Example 3: Cesium Chloride (Cs. Cl) radius: Cs+ = 1. 74 nm, Cl- = 1. 81 nm AL Chemistry radius ratio = 0. 960 SC ► Anions occupy the corners of a unit cell, the centre of the cube is larger than the tetrahedral and octahedral sites, therefore the large Cs+ ion can fit in. p. 15

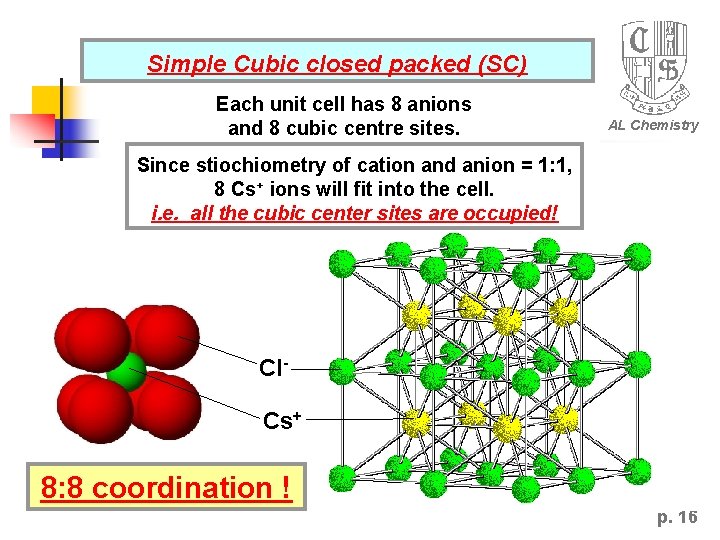
Simple Cubic closed packed (SC) Each unit cell has 8 anions and 8 cubic centre sites. AL Chemistry Since stiochiometry of cation and anion = 1: 1, 8 Cs+ ions will fit into the cell. i. e. all the cubic center sites are occupied! Cl. Cs+ 8: 8 coordination ! p. 16

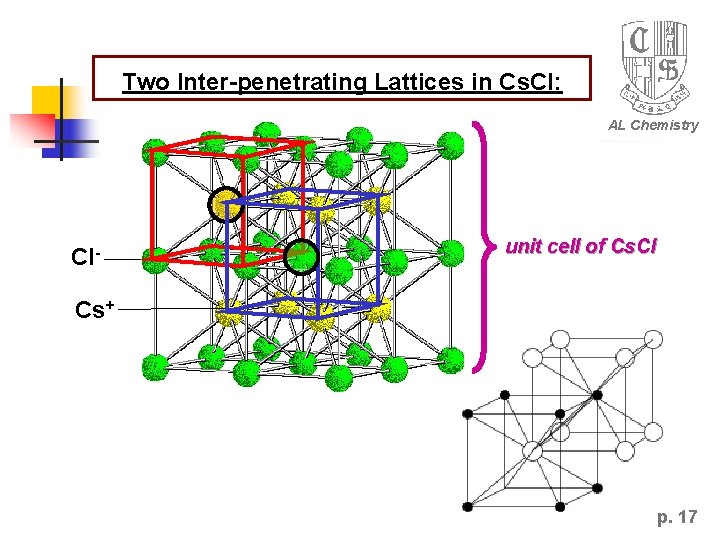
Two Inter-penetrating Lattices in Cs. Cl: AL Chemistry Cl- unit cell of Cs. Cl Cs+ p. 17

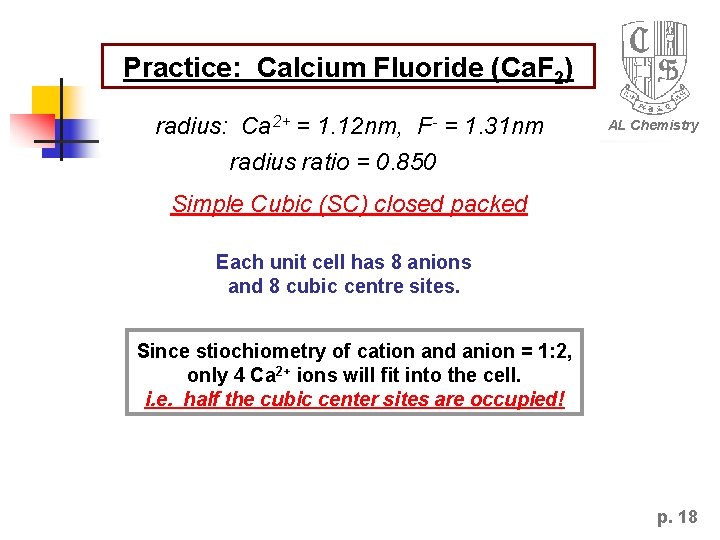
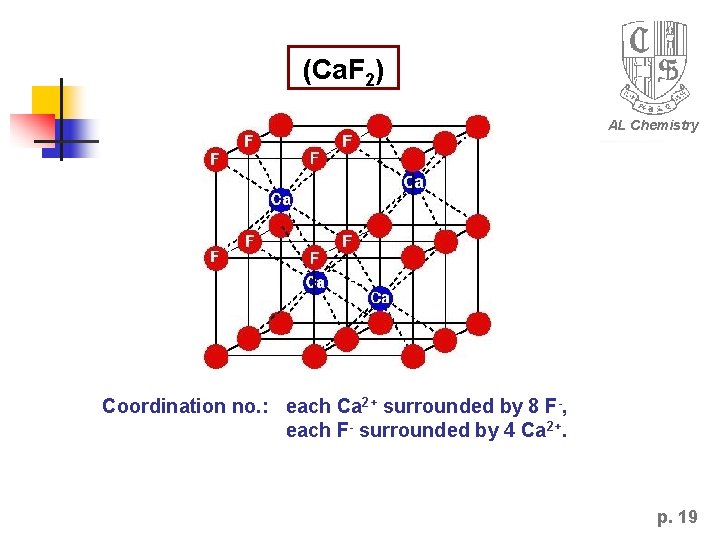
Practice: Calcium Fluoride (Ca. F 2) radius: Ca 2+ = 1. 12 nm, F- = 1. 31 nm AL Chemistry radius ratio = 0. 850 Simple Cubic (SC) closed packed Each unit cell has 8 anions and 8 cubic centre sites. Since stiochiometry of cation and anion = 1: 2, only 4 Ca 2+ ions will fit into the cell. i. e. half the cubic center sites are occupied! p. 18

(Ca. F 2) AL Chemistry Coordination no. : each Ca 2+ surrounded by 8 F-, each F- surrounded by 4 Ca 2+. p. 19

Conclusion …. . Closed packed of Anions & Cation: AL Chemistry if the cation is small if the cation is not small anions are packed in form of “FCC” anions are packed in form of “SC” cations fill into “tetrahedral holes” cations fill into “octahedral holes” e. g. Na. Cl e. g. Zn. S cations fill into the “cubic centre site” e. g. Cs. Cl, Ca. F 2 p. 20