Unit B Atoms Electrons and the Periodic Table
Unit B Atoms, Electrons and the Periodic Table

B. 1 A Look Inside Matter n Since the days of the ancient Greeks people have wondered about matter: Ø Ø Is matter capable of being broken into infinitely small particles or is there a basic building block – a simplest particle to an element? (The word atom comes from the Greek word átomos meaning “indivisible. ”) How can the properties of matter be explained?

B. 1 A Look Inside Matter n n The following sections will help to explain why elements have the properties that are observed. The Atomic Theory will show the facts are easily understood by using various models that have been refined over the years as new observations are discovered.
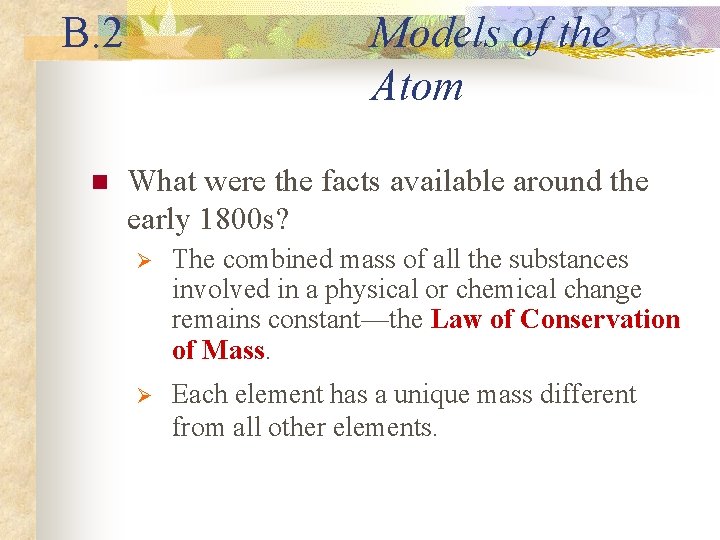
B. 2 n Models of the Atom What were the facts available around the early 1800 s? Ø The combined mass of all the substances involved in a physical or chemical change remains constant—the Law of Conservation of Mass. Ø Each element has a unique mass different from all other elements.
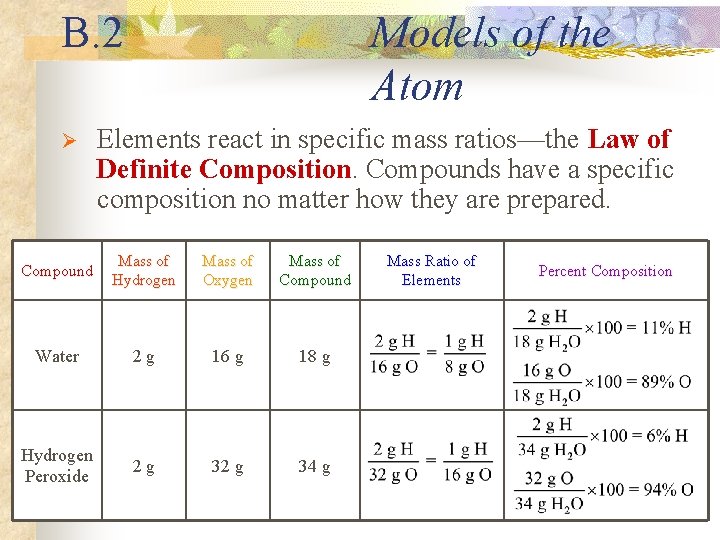
B. 2 Ø Models of the Atom Elements react in specific mass ratios—the Law of Definite Composition. Compounds have a specific composition no matter how they are prepared. Compound Mass of Hydrogen Mass of Oxygen Mass of Compound Water 2 g 16 g 18 g Hydrogen Peroxide 2 g 32 g 34 g Mass Ratio of Elements Percent Composition
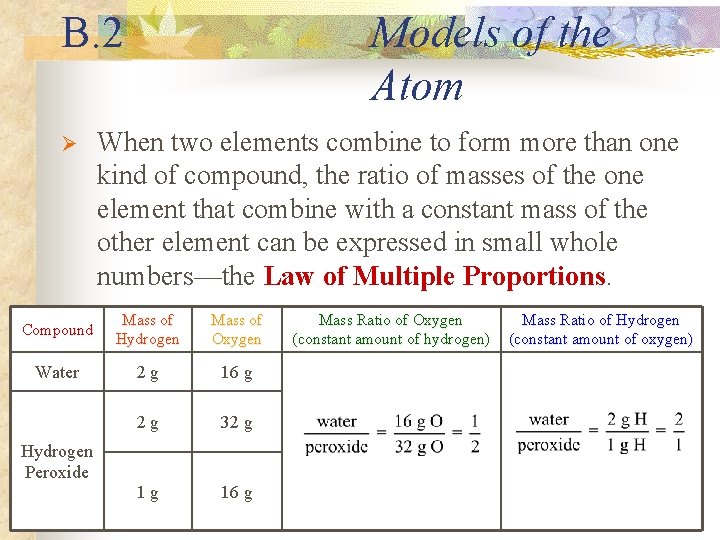
B. 2 Ø Models of the Atom When two elements combine to form more than one kind of compound, the ratio of masses of the one element that combine with a constant mass of the other element can be expressed in small whole numbers—the Law of Multiple Proportions. Compound Mass of Hydrogen Mass of Oxygen Water 2 g 16 g 2 g 32 g 1 g 16 g Hydrogen Peroxide Mass Ratio of Oxygen (constant amount of hydrogen) Mass Ratio of Hydrogen (constant amount of oxygen)
B. 2 n Models of the Atom Dalton Model Ø Elements are made up of indivisible, spherical particles called atoms. Figure B 1 – “Billiard Ball” Model
B. 2 n Models of the Atom Additional Assumptions (Dalton Model): Ø Atoms of one substance are chemically alike and have the same mass. Ø Only atoms can work together in a reaction. Compounds contain two or more atoms joined together. Chemical reactions consist of rearranging atoms. Ø Ø
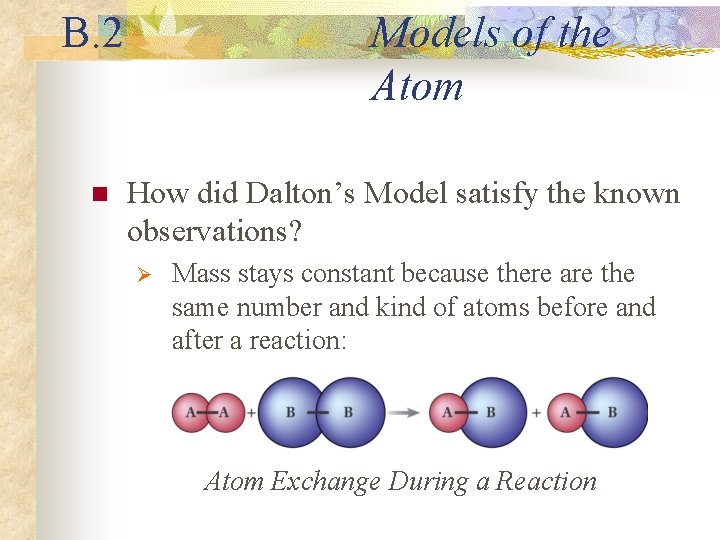
B. 2 n Models of the Atom How did Dalton’s Model satisfy the known observations? Ø Mass stays constant because there are the same number and kind of atoms before and after a reaction: Atom Exchange During a Reaction
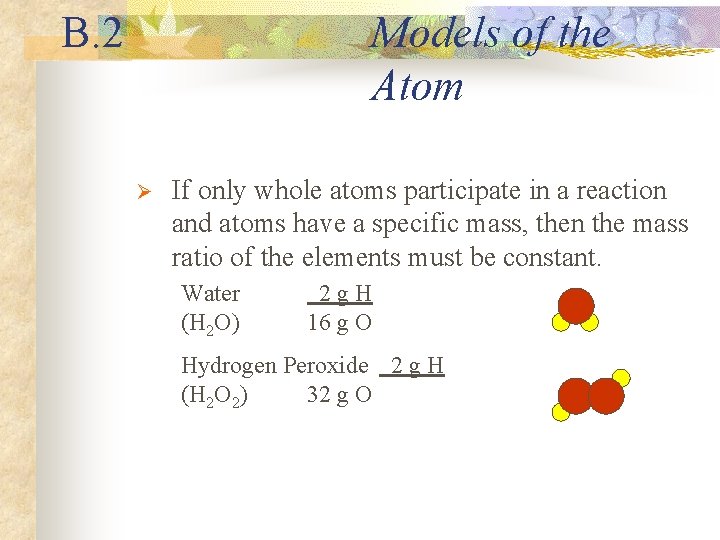
B. 2 Models of the Atom Ø If only whole atoms participate in a reaction and atoms have a specific mass, then the mass ratio of the elements must be constant. Water (H 2 O) 2 g. H 16 g O Hydrogen Peroxide 2 g H (H 2 O 2) 32 g O
- Slides: 10