Unit Atomic Structure History of the Atom Important







- Slides: 16

Unit: Atomic Structure

History of the Atom Important Experiments Leading to Atomic Theory

Democritus (400 B. C. ) • A Greek philosopher • Was the first person to think about an atom’s existence. • Believed that matter was composed of tiny indivisible particles called atoms. Hmmm… atoms… “atomos” • He had no experimental evidence to support his thoughts.

John Dalton (1766 -1844) • A meteorologist • Unlike Democritus, he had experimental evidence to support his theory. • Dalton had four major points (postulates) to his theory.

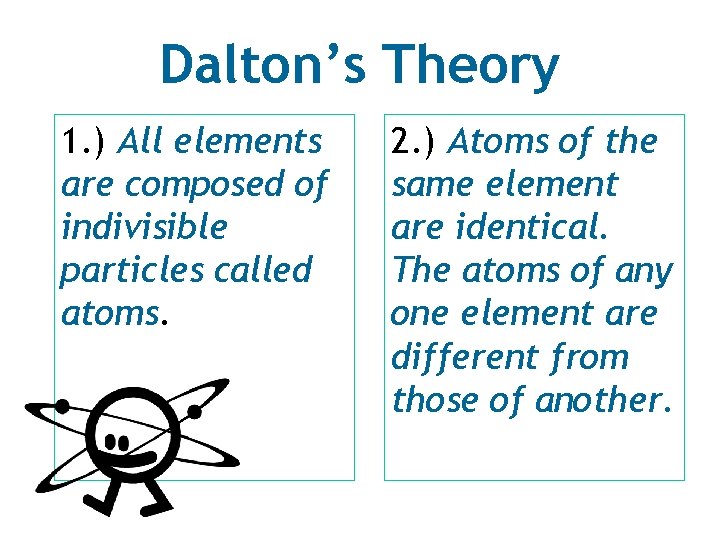
Dalton’s Theory 1. ) All elements are composed of indivisible particles called atoms. 2. ) Atoms of the same element are identical. The atoms of any one element are different from those of another.

Dalton’s Theory 3. ) Atoms of different elements mix or combine in whole number ratios. Example: Oxygen combines with hydrogen to form water in a 2: 1 ratio. 4. ) Chemical reactions occur when atoms separate, join, or rearrange. In a chemical reaction, atoms of one element NEVER change into another.

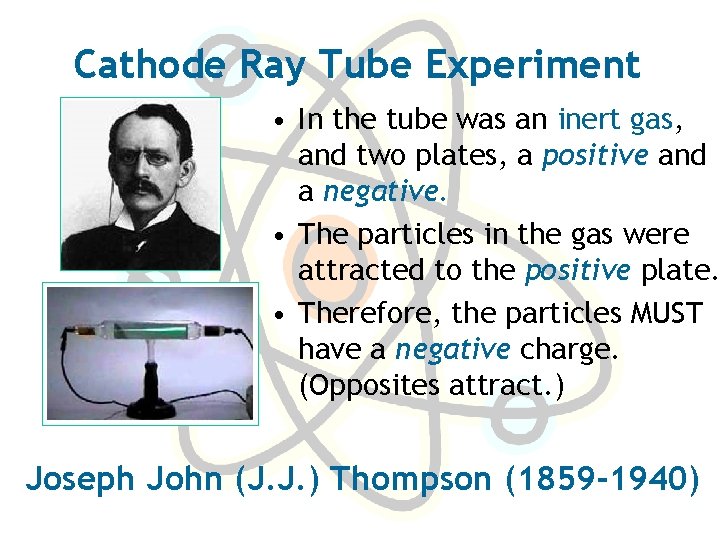
Cathode Ray Tube Experiment • In the tube was an inert gas, and two plates, a positive and a negative. • The particles in the gas were attracted to the positive plate. • Therefore, the particles MUST have a negative charge. (Opposites attract. ) Joseph John (J. J. ) Thompson (1859 -1940)

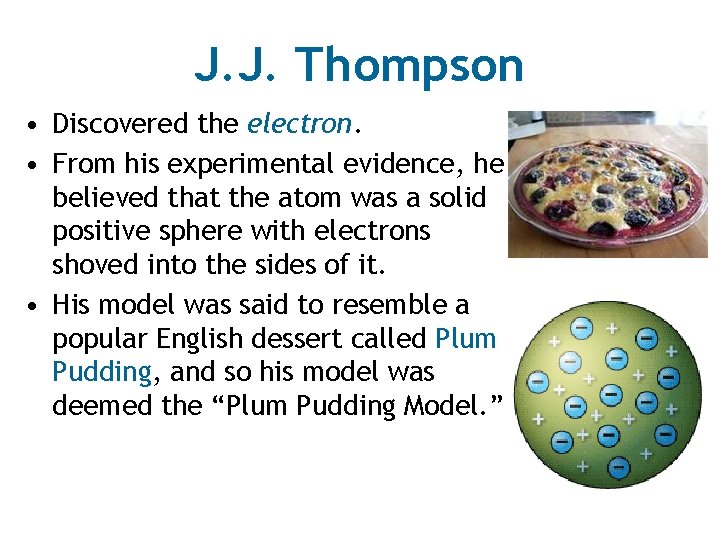
J. J. Thompson • Discovered the electron. • From his experimental evidence, he believed that the atom was a solid positive sphere with electrons shoved into the sides of it. • His model was said to resemble a popular English dessert called Plum Pudding, and so his model was deemed the “Plum Pudding Model. ”

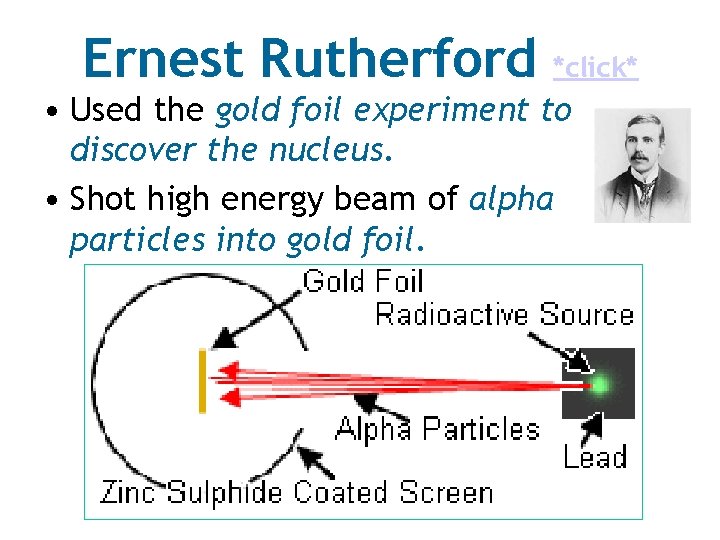
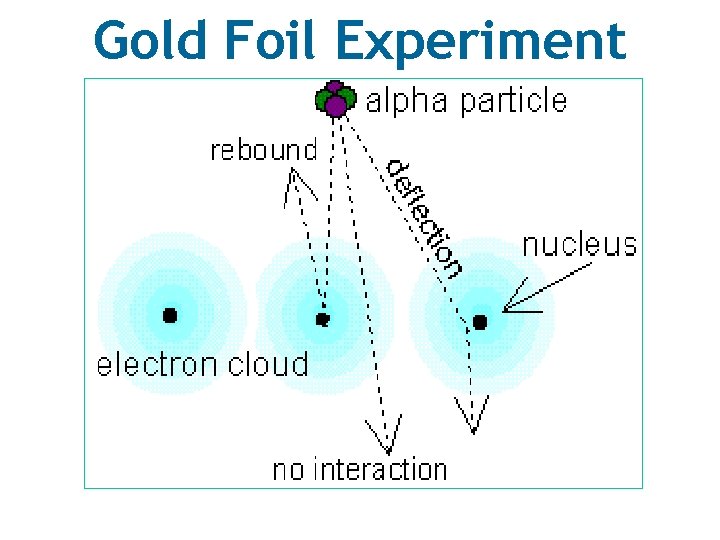
Ernest Rutherford *click* • Used the gold foil experiment to discover the nucleus. • Shot high energy beam of alpha particles into gold foil.

Gold Foil Experiment

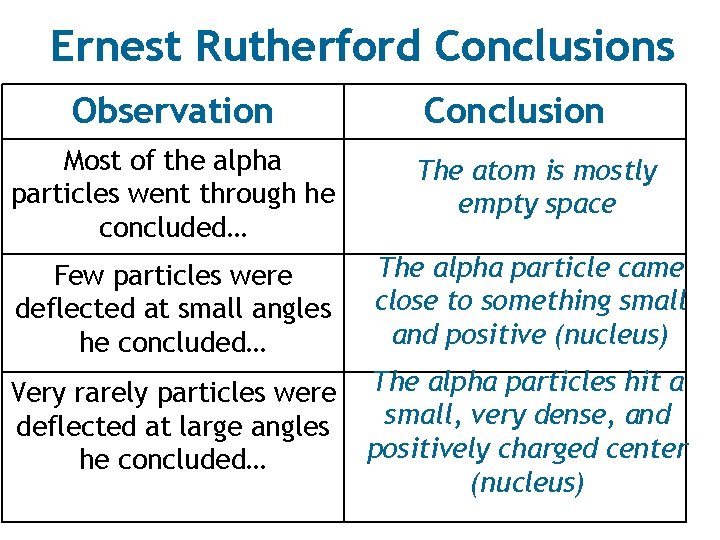
Ernest Rutherford Conclusions Observation Conclusion Most of the alpha particles went through he concluded… The atom is mostly empty space Few particles were deflected at small angles he concluded… The alpha particle came close to something small and positive (nucleus) Very rarely particles were deflected at large angles he concluded… The alpha particles hit a small, very dense, and positively charged center (nucleus)

Eugene Goldstein (1850 -1930) • Goldstein discovered the proton. James Chadwick (1891 -1974) • Chadwick discovered the neutron.

Side note… Not all of Dalton’s postulates were correct. • We now know that atoms are indeed divisible – atoms can be broken down into their subatomic particles, protons, neutrons, and electrons (and these too can be broken down even further!). • We also know that not all atoms of the same element are identical. Isotopes exist for different elements. (We’ll talk about this later. )

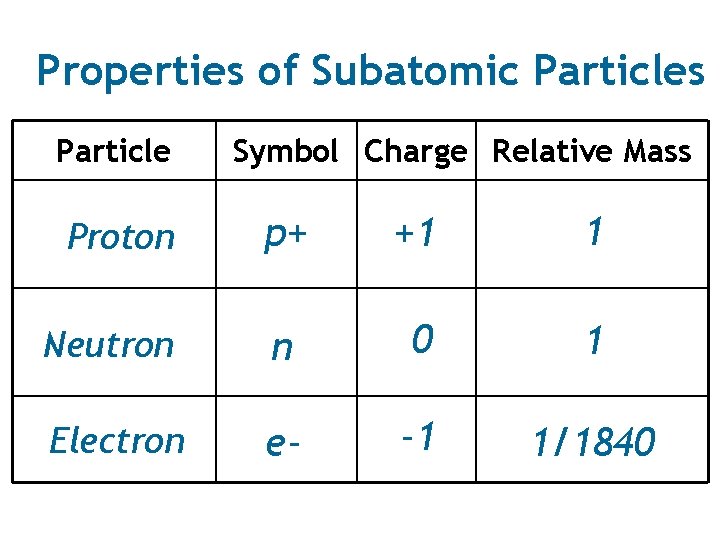
Properties of Subatomic Particles Particle Symbol Charge Relative Mass p+ +1 1 Neutron n 0 1 Electron e- -1 1/1840 Proton

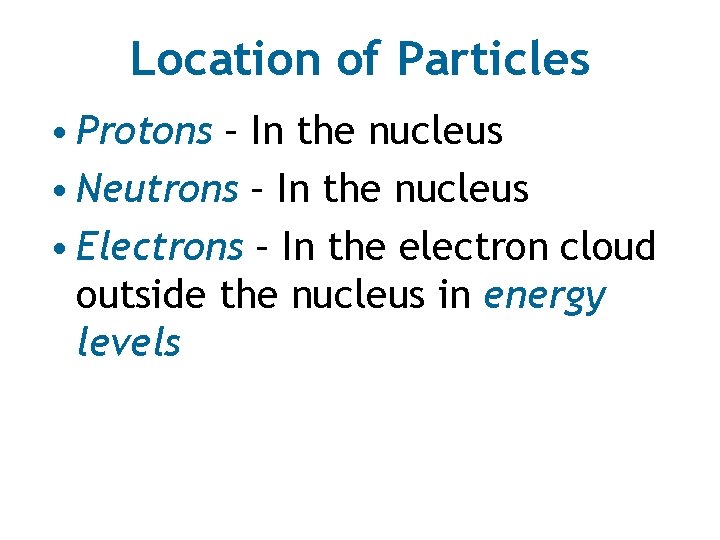
Location of Particles • Protons – In the nucleus • Neutrons – In the nucleus • Electrons – In the electron cloud outside the nucleus in energy levels

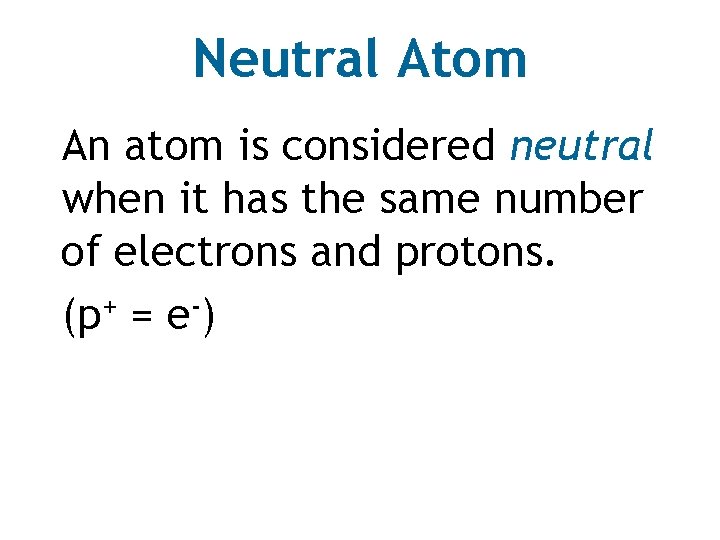
Neutral Atom An atom is considered neutral when it has the same number of electrons and protons. (p+ = e-)