Unit 9 The Mole What is a mole







- Slides: 13

Unit 9 - The Mole What is a mole? One Step Problems

What is a mole? • A furry, burrowing woodland creature, revered by many and adored by all? Maybe! • But not really in chemistry!

A Mole. . . • is a unit of measurement that tells how many particles of something you have!

• much like a dozen (12), a gross (144) or a ream (500) is just a unit that groups smaller numbers into big ones. . . but its a big one; a REALLY big one!

1 mole = 6. 02 x 1023 particles! (that’s a lot of flowers!)

What are particles? • Particles could be anything, but because of the size of the number, we usually mean Atoms, molecules, or formula units.

Avogadro’s Number • The number 6. 02 x 1023 is called Avogadro’s number. • This is due to Amadeo Avagadro’s research leading to the discovery, not the discovery of the number itself. It is a little known fact that Avogadro was voted his class’s most likely to have a scientific discovery and most likely to look really creepy.

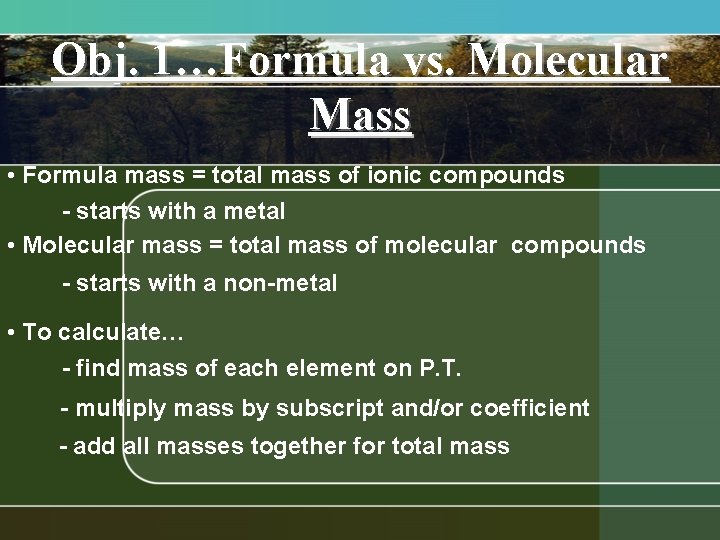
Obj. 1…Formula vs. Molecular Mass • Formula mass = total mass of ionic compounds - starts with a metal • Molecular mass = total mass of molecular compounds - starts with a non-metal • To calculate… - find mass of each element on P. T. - multiply mass by subscript and/or coefficient - add all masses together for total mass

Obj. 1 cont… • Practice… S 3 F 6 19. 00 x 6 = 114. 00 32. 07 x 3 = 96. 21 + 114. 00 = 210. 21 amu Al 2(SO 4)3 26. 98 x 2 = 53. 96 16. 00 x 12 = 192. 00 32. 07 x 3 = 96. 21 53. 96 + 96. 21 + 192. 00= 342. 19 amu

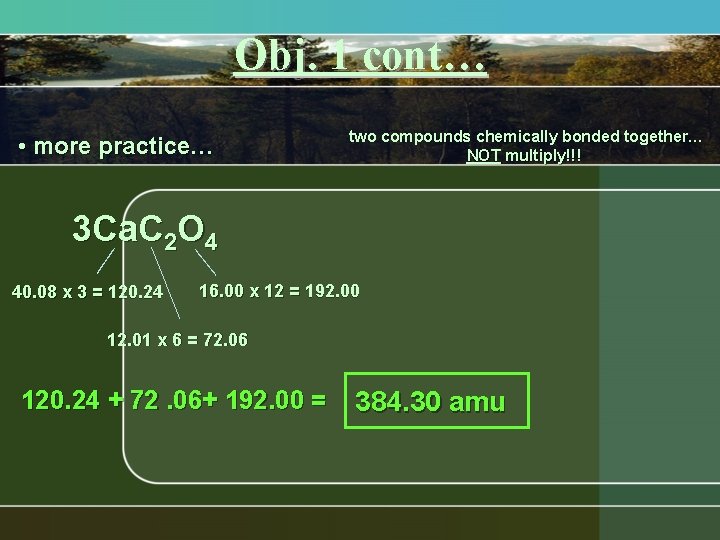
Obj. 1 cont… • more practice… two compounds chemically bonded together… NOT multiply!!! 3 Ca. C 2 O 4 40. 08 x 3 = 120. 24 16. 00 x 12 = 192. 00 12. 01 x 6 = 72. 06 120. 24 + 72. 06+ 192. 00 = 384. 30 amu

Obj. 2 cont… • recall that one p+ / n 0 = 1. 67 x 10 -24 grams • therefore…one mole of nucleons = 1 gram ** 1. 67 E -24 x 6. 02 E 23 = 1 • amu = g/mole atomic mass molar mass • one mole = ~ 6. 02 x 1023 atoms, molecules or ions in a compound ~ molar mass of compound / element ~ 22. 4 liters of any gas at STP (standard temp. and pressure) 0°C 273 K 760 mm. Hg 101. 3 k. Pa 1 atm

Mole Map

Obj. 3 -7 cont… • Practice, practice… 2. 0 moles He = ______ atoms He 2 16 = 18 2. 0 moles H 2 O = ______ grams H 2 O 67. 2 liters O 2 = ______ moles O 2 2 16 = 18 27 grams H 2 O = ______ moles H 2 O