Unit 9 Gases 1 Pressure Gas pressure the













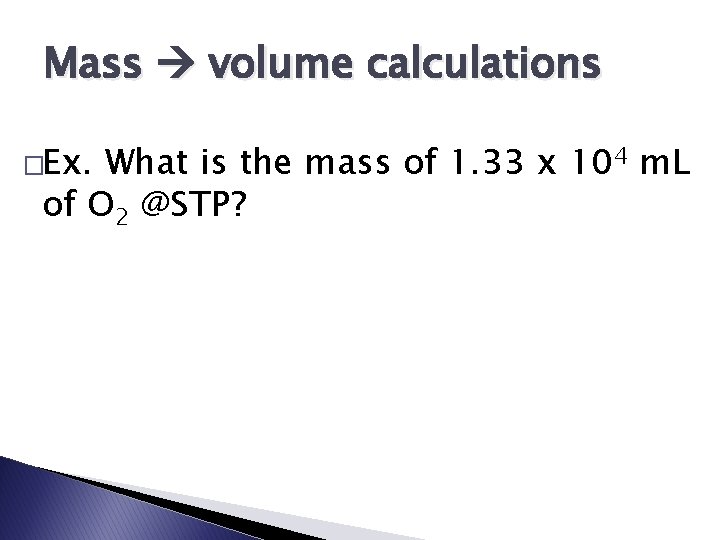








- Slides: 45

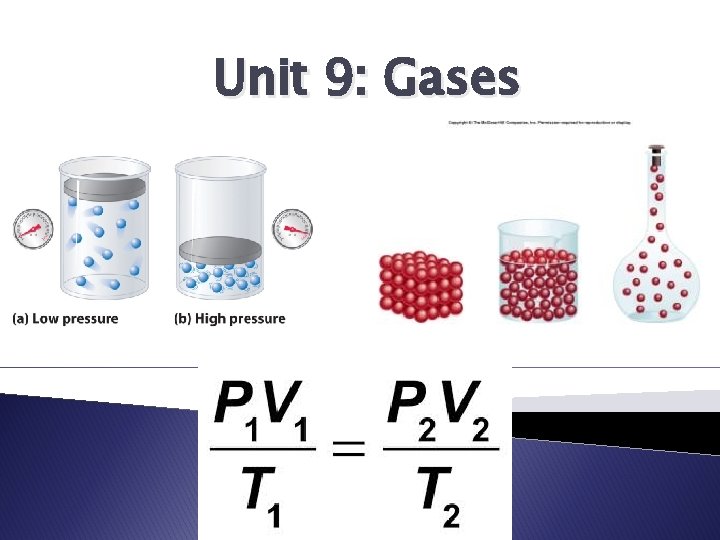
Unit 9: Gases

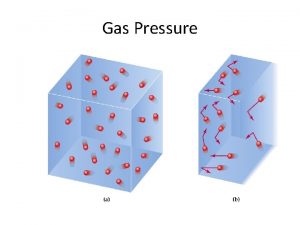
1. Pressure • • • Gas pressure: the result of collisions of billions of rapidly moving gas particles with other objects • Atmospheric pressure results from the collisions of atoms and molecules in air with objects SI unit of pressure: Pascal (Pa) Other units include • millimeters of mercury: mm. Hg • atmospheres: atm • torr

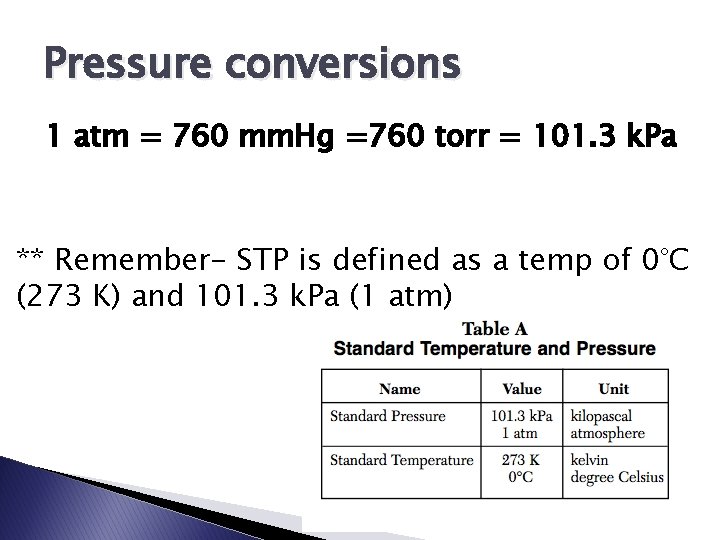
Pressure conversions 1 atm = 760 mm. Hg =760 torr = 101. 3 k. Pa ** Remember- STP is defined as a temp of 0°C (273 K) and 101. 3 k. Pa (1 atm)

Practice A pressure gauge records a pressure of 89. 0 k. Pa. What is this measurement in a) atmospheres? b) mm. Hg? 1 atm = 760 mm. Hg = 101. 3 k. Pa

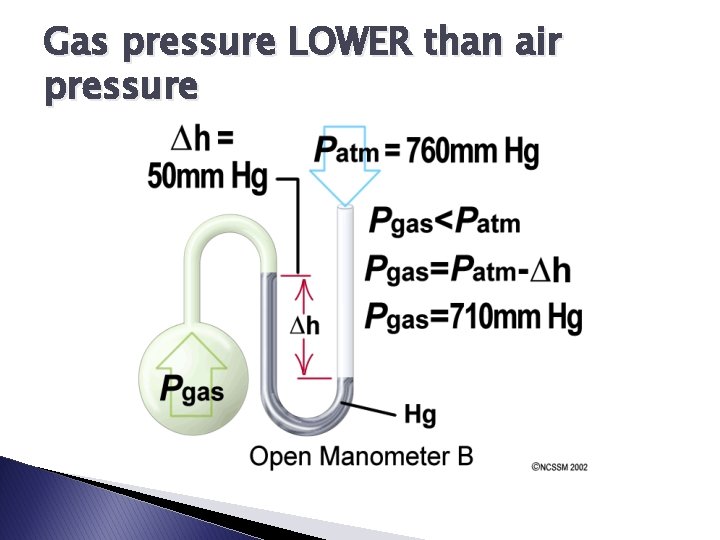
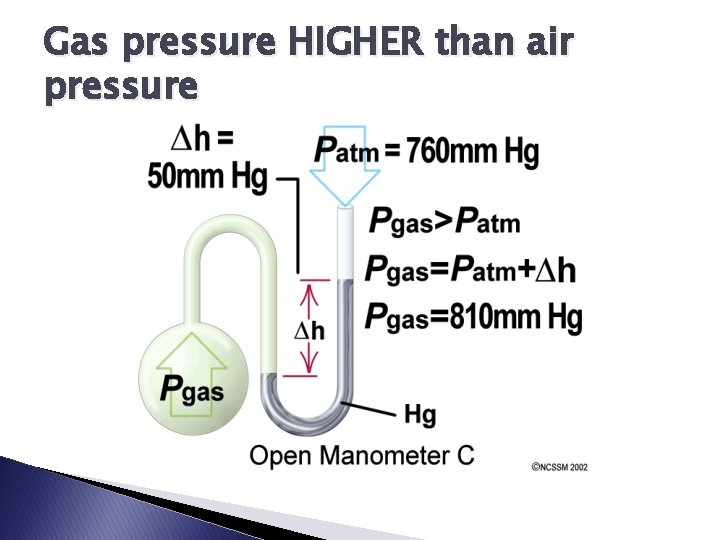
How do we measure the pressure of any gas? consists of a U shaped glass tube filled with some liquid (typically Hg due to its high density) � MANOMETER:

Gas pressure LOWER than air pressure

Gas pressure HIGHER than air pressure

2. Volume • Volume is a measure of how much space an object takes up • SI unit for volume = Liter • Remember: 1 L = 1000 m. L

3. Temperature • Temperature is a measure of the average kinetic energy of the particles in a substance • SI unit for temp: Kelvin • Remember: K = °C + 273

The Gas Laws � Show relationships between pressure, volume and temperature for an ideal gas � 3 major gas laws **All temperatures MUST be in Kelvins.

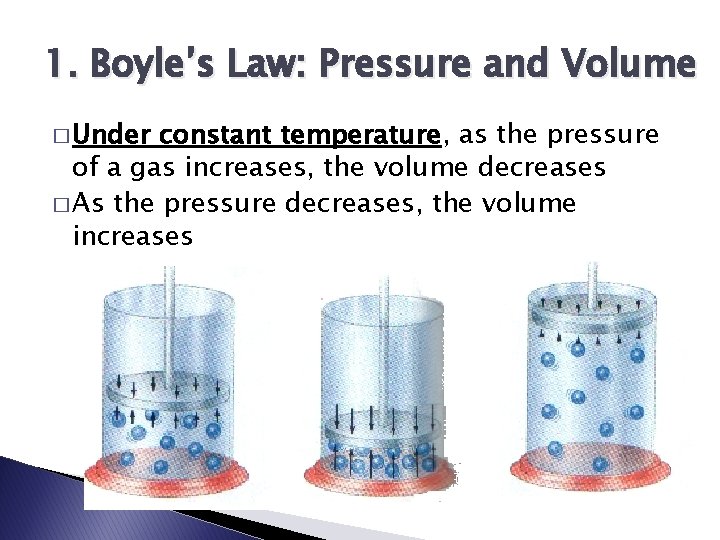
1. Boyle’s Law: Pressure and Volume � Under constant temperature, as the pressure of a gas increases, the volume decreases � As the pressure decreases, the volume increases

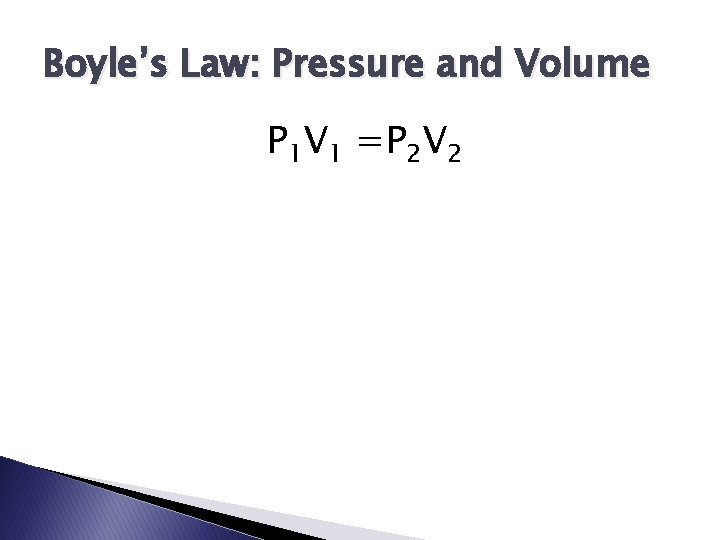
Boyle’s Law: Pressure and Volume P 1 V 1 =P 2 V 2

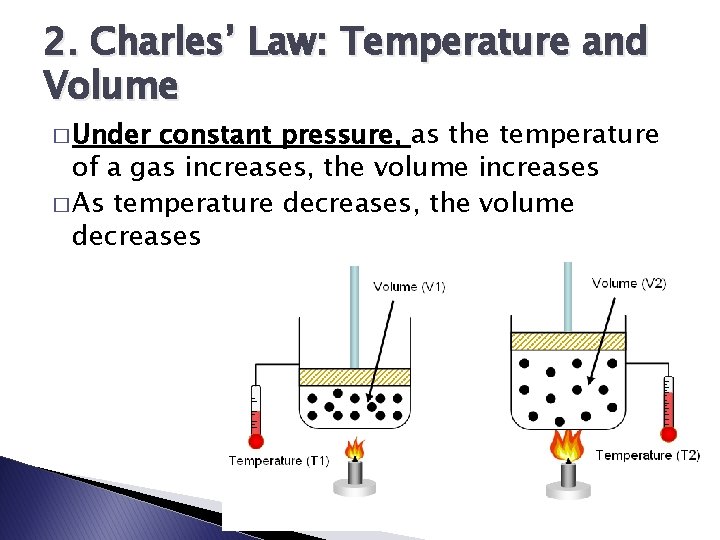
2. Charles’ Law: Temperature and Volume � Under constant pressure, as the temperature of a gas increases, the volume increases � As temperature decreases, the volume decreases

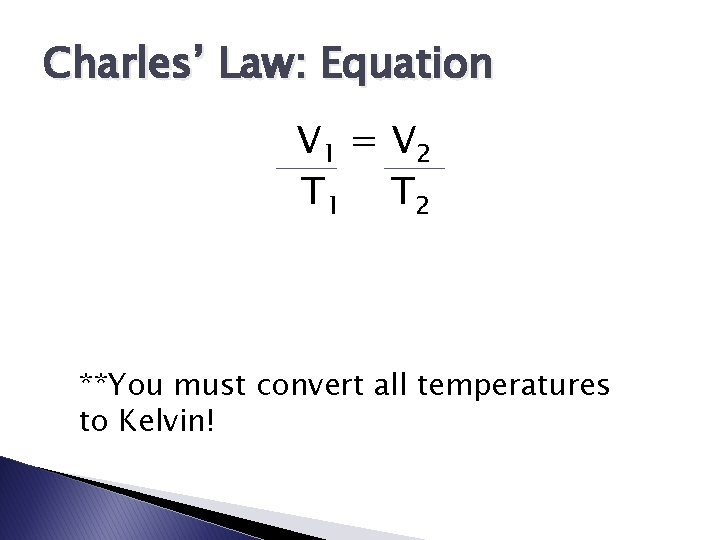
Charles’ Law: Equation V 1 = V 2 T 1 T 2 **You must convert all temperatures to Kelvin!

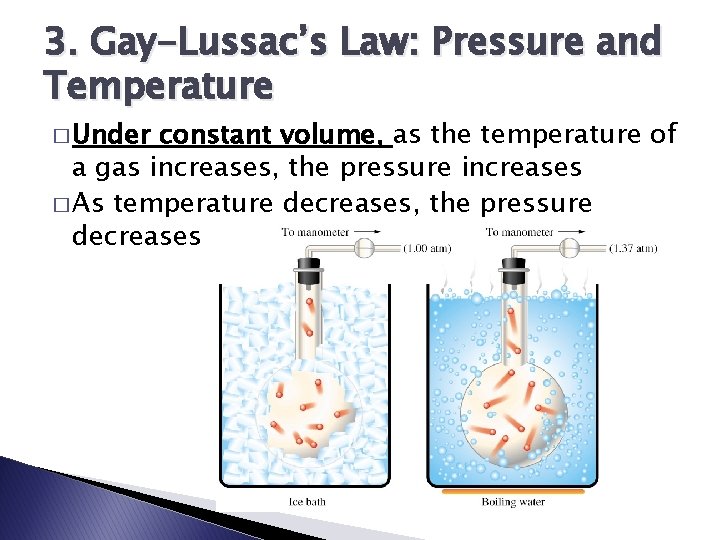
3. Gay-Lussac’s Law: Pressure and Temperature � Under constant volume, as the temperature of a gas increases, the pressure increases � As temperature decreases, the pressure decreases

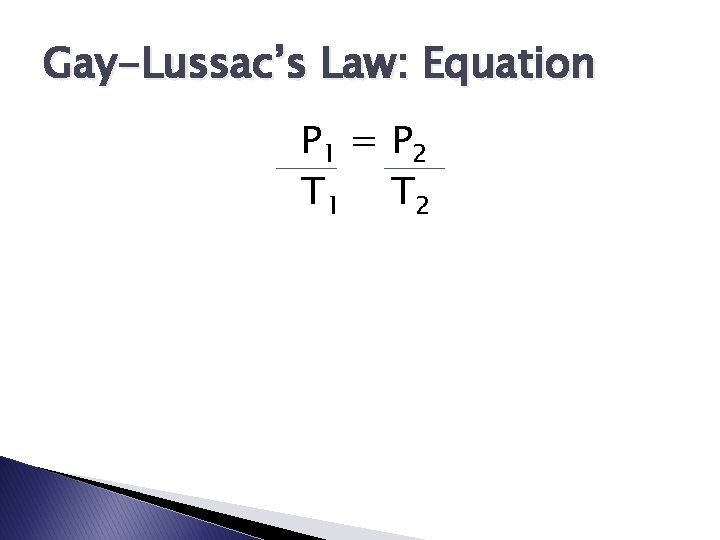
Gay-Lussac’s Law: Equation P 1 = P 2 T 1 T 2

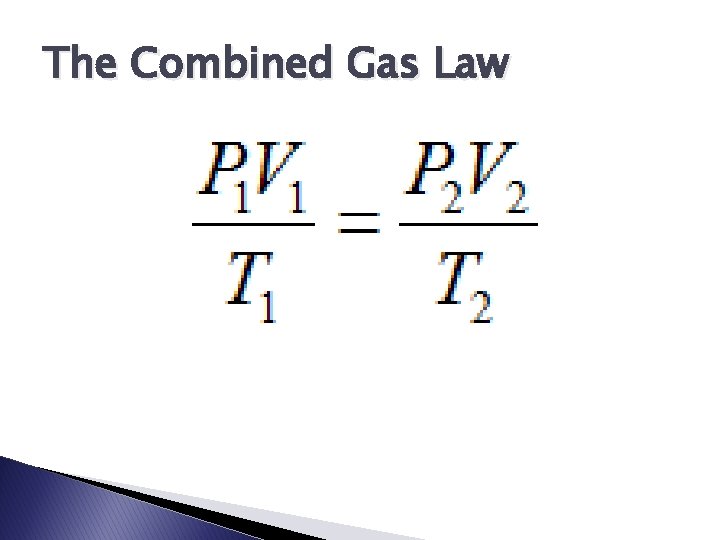
The Combined Gas Law

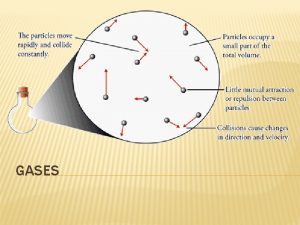
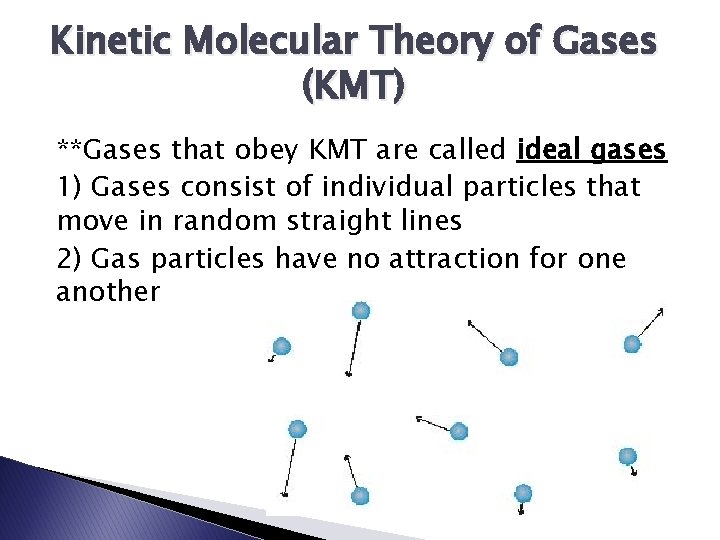
Kinetic Molecular Theory of Gases (KMT) **Gases that obey KMT are called ideal gases 1) Gases consist of individual particles that move in random straight lines 2) Gas particles have no attraction for one another

Kinetic Molecular Theory of Gases (KMT) 3) Gas particles are separated by such large distances that the particles themselves have negligible volume 4) When gas particles collide, the collisions are perfectly elastic (particles may transfer energy, but there is no net loss of energy)

When do gases behave most like ideal gases? � Under conditions of low pressure and high temperature � Lighter (smaller) gases behave more like ideal gases than heavier ones

These ideal “laws” are OK for molecules that are: �Small �Nonpolar ***2 real gases that behave most ideal: �H 2 and He

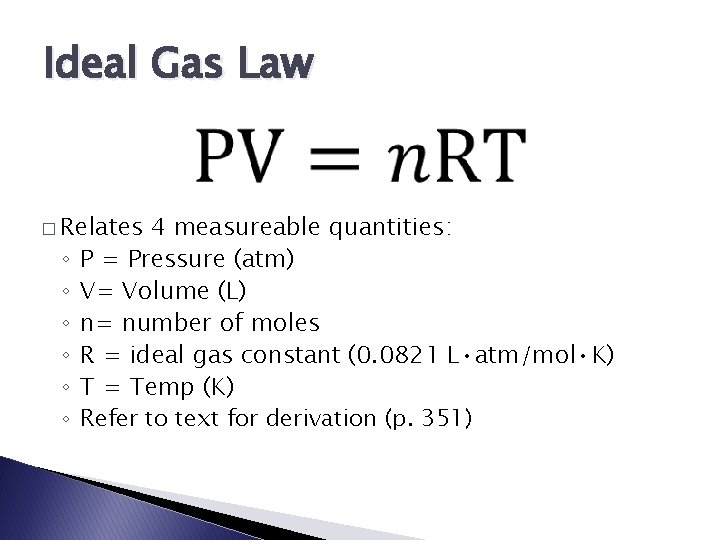
Ideal Gas Law � Relates ◦ ◦ ◦ 4 measureable quantities: P = Pressure (atm) V= Volume (L) n= number of moles R = ideal gas constant (0. 0821 L • atm/mol • K) T = Temp (K) Refer to text for derivation (p. 351)

Ideal Gas Law- Examples �What is the pressure, in atm, of a. 500 mol sample of N 2(g) in a 10. 0 L container at 298 K? �How many grams of O 2 are in a 125 m. L container at 45°C and 340. mm. Hg?

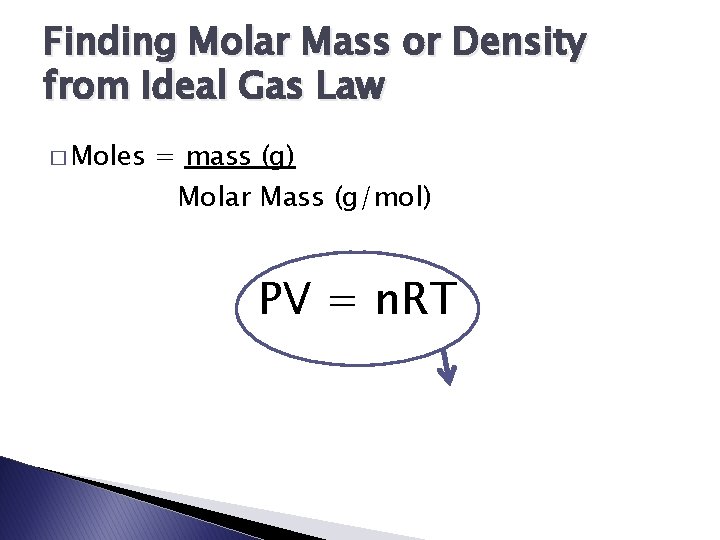
Finding Molar Mass or Density from Ideal Gas Law � Moles = mass (g) Molar Mass (g/mol) PV = n. RT

Finding Molar Mass or Density from Ideal Gas Law �PV = m. RT M Rearranged: M= m. RT PV

Since �d = m v M= m. RT = PV Rearranged: d. RT P d = MP RT

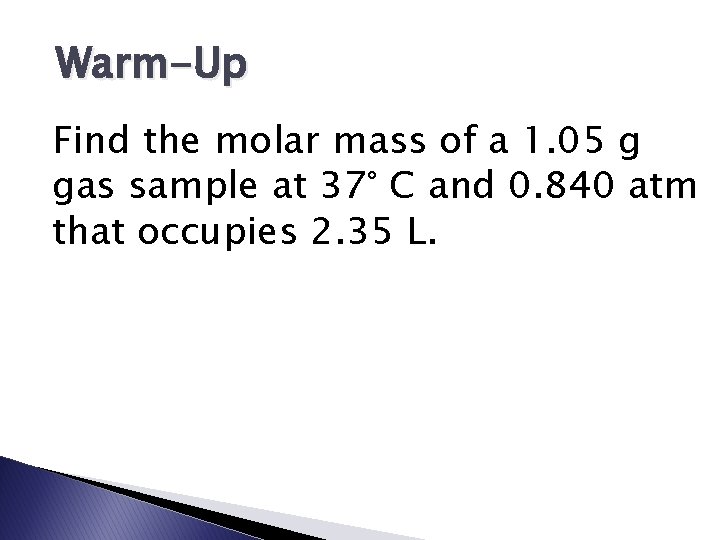
Warm-Up Find the molar mass of a 1. 05 g gas sample at 37° C and 0. 840 atm that occupies 2. 35 L.

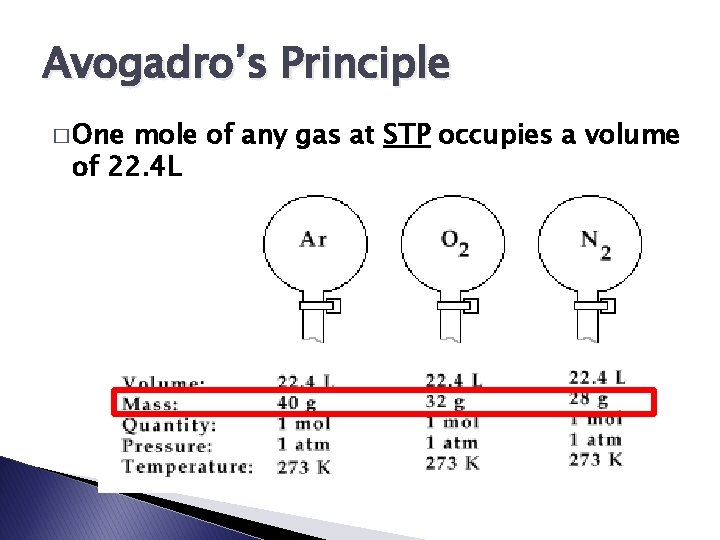
Avogadro’s Principle � One mole of any gas at STP occupies a volume of 22. 4 L

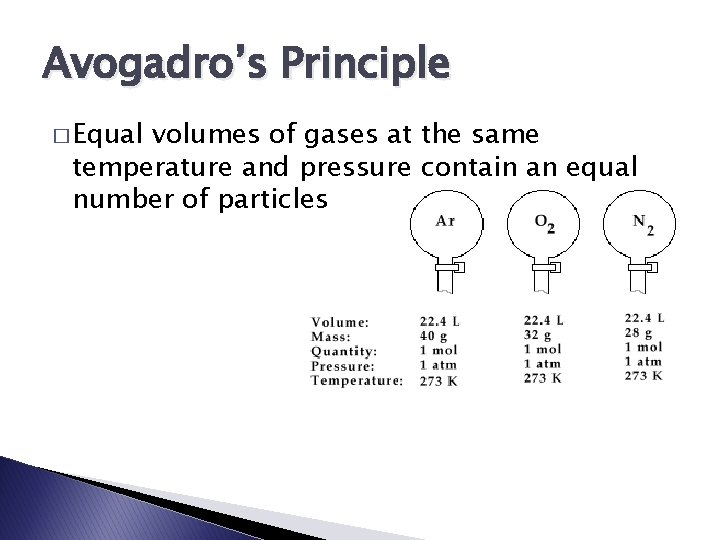
Avogadro’s Principle � Equal volumes of gases at the same temperature and pressure contain an equal number of particles

mol volume calculations 1 mol = 22. 4 L at STP Assuming STP: � Convert 0. 42 mol H 2 (g) to liters � Convert 64. 3 L O 2 (g) to moles

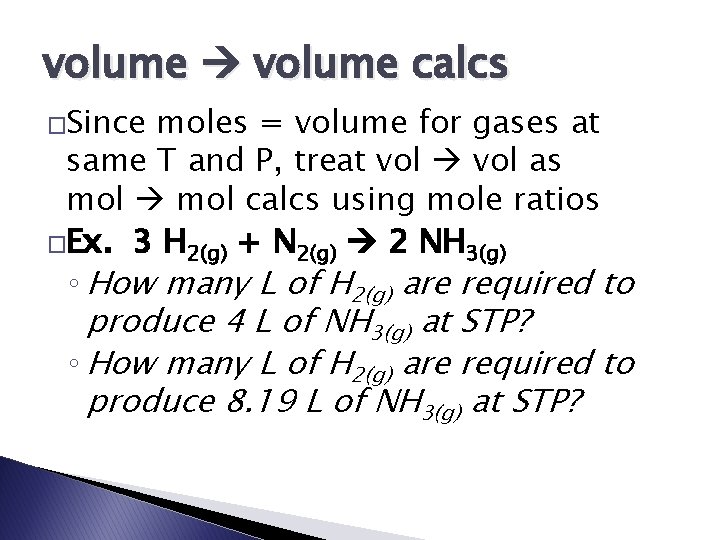
volume calcs �Since moles = volume for gases at same T and P, treat vol as mol calcs using mole ratios �Ex. 3 H 2(g) + N 2(g) 2 NH 3(g) ◦ How many L of H 2(g) are required to produce 4 L of NH 3(g) at STP? ◦ How many L of H 2(g) are required to produce 8. 19 L of NH 3(g) at STP?
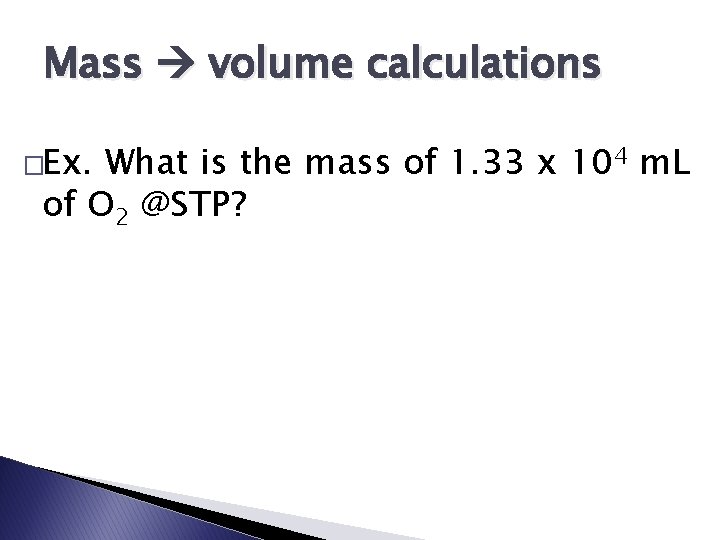
Mass volume calculations �Ex. What is the mass of 1. 33 x 104 m. L of O 2 @STP?

Gas Density �Usually given in g/L @ STP �Density (@STP) = Molar Mass (g/mol) Molar Volume (22. 4 L/mol)

Example �What is the density of CO 2(g), in g/L @STP?

Warm-Up �A 0. 519 g gas sample is found to have a V= 200. m. L @STP. What is the molar mass of this gas? ◦ Molar Mass = Density * Molar Volume

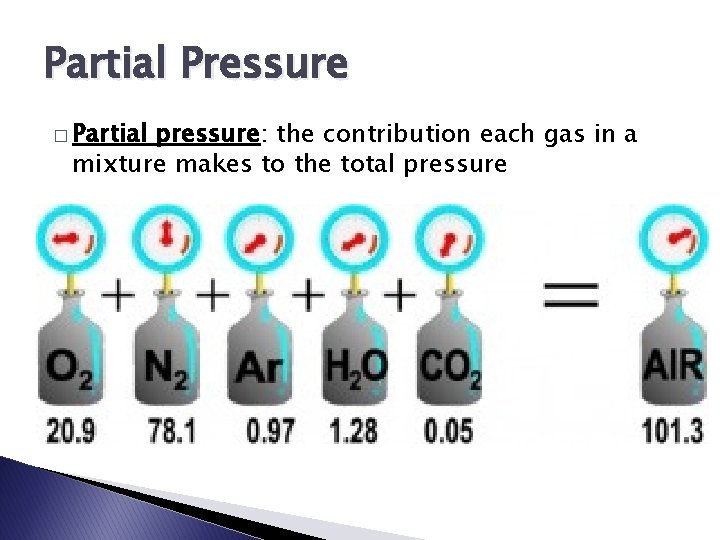
Partial Pressure � Partial pressure: the contribution each gas in a mixture makes to the total pressure

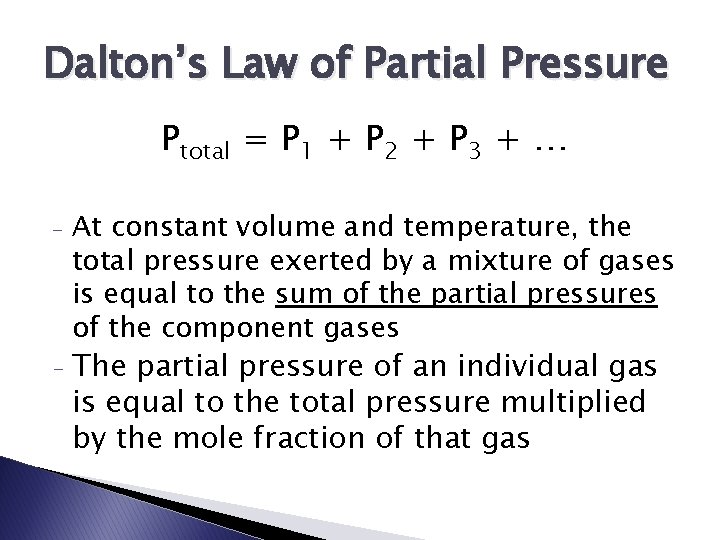
Dalton’s Law of Partial Pressure Ptotal = P 1 + P 2 + P 3 + … - - At constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the component gases The partial pressure of an individual gas is equal to the total pressure multiplied by the mole fraction of that gas

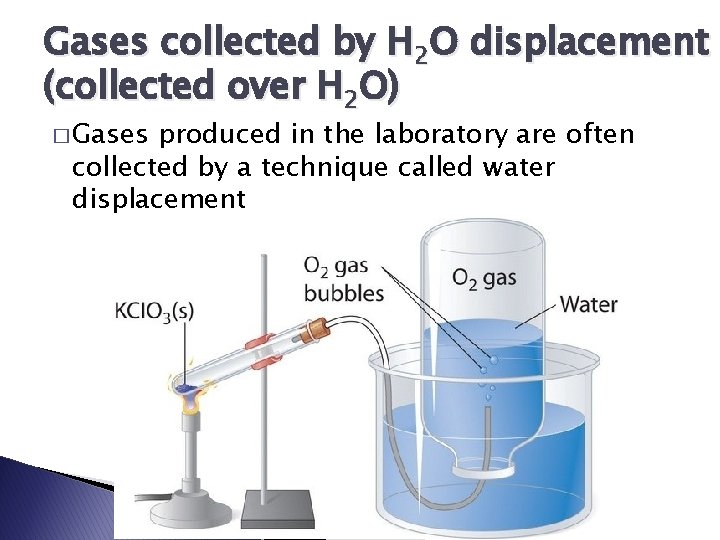
Gases collected by H 2 O displacement (collected over H 2 O) � Gases produced in the laboratory are often collected by a technique called water displacement

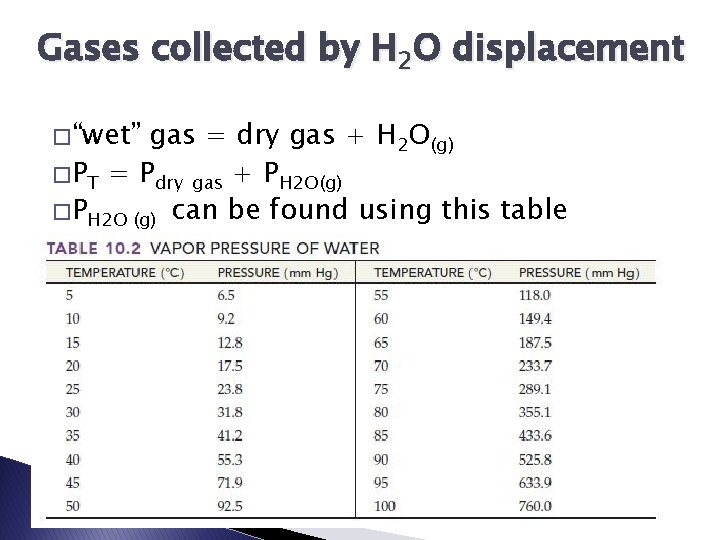
Gases collected by H 2 O displacement � “wet” gas = dry gas + H 2 O(g) � PT = Pdry gas + PH 2 O(g) � PH 2 O (g) can be found using this table

Graham’s Law � Diffusion: the tendency of molecules to move from higher to lower concentration � Effusion: when a gas escapes through a tiny hole in its container

Graham’s Law Qualitatively… �Smaller, lighter gas molecules (lower molar mass) will diffuse faster than bigger, heavier gas molecules.

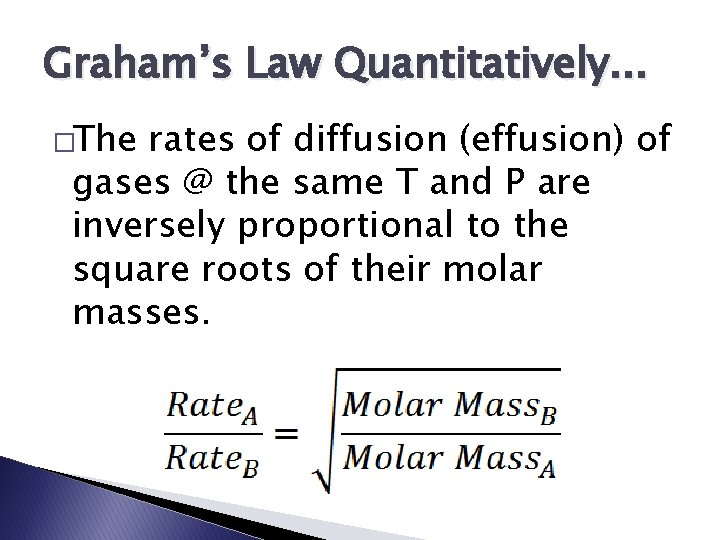
Graham’s Law Quantitatively. . . �The rates of diffusion (effusion) of gases @ the same T and P are inversely proportional to the square roots of their molar masses.

Example �Compare H 2(g) rates of diffusion of and O 2(g).

Because density varies directly with Molar Mass… � rate of diffusion of A = √density B rate of diffusion of B √density A

Real vs. Ideal gases � Particles of a real gas ARE attracted to one another (and can condense) � Real gas particles DO have volume � When gas particles collide, the collisions are NOT perfectly elastic (there is some loss of energy)
 How does a gas exert pressure
How does a gas exert pressure Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Unit 24 cutting with oxyfuels and other gases
Unit 24 cutting with oxyfuels and other gases Unit 6 review questions
Unit 6 review questions Derive ideal gas equation
Derive ideal gas equation Imaginary gas
Imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Sutherland's law
Sutherland's law Conclusion bhopal gas tragedy
Conclusion bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Gas reale e gas ideale
Gas reale e gas ideale Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Hukum laju terintegrasi
Hukum laju terintegrasi Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Gaya yang besarnya 50 dyne menimbulkan tekanan
Gaya yang besarnya 50 dyne menimbulkan tekanan What gas law relates pressure and temperature
What gas law relates pressure and temperature Partial pressure
Partial pressure A gas has a pressure of 699 mmhg at 40
A gas has a pressure of 699 mmhg at 40 Gas pressure units
Gas pressure units Partial pressure of a gas
Partial pressure of a gas 13-4 practice problems chemistry
13-4 practice problems chemistry Grahams law
Grahams law In hptlc gas used as pressure on linomat
In hptlc gas used as pressure on linomat Gas pressure physics
Gas pressure physics A gas has a pressure of 710 kpa at 227
A gas has a pressure of 710 kpa at 227 Pressure is defined as
Pressure is defined as Calculate the density of xenon gas at a pressure of 742
Calculate the density of xenon gas at a pressure of 742 Ideal gas law pressure formula
Ideal gas law pressure formula Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi
Hát lên người ơi Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết