Unit 9 Covalent Bonding Chapters 8 9 Chemistry

Unit 9: Covalent Bonding Chapters 8 & 9 Chemistry 1 L Cypress Creek High School

WARNING!!!!!!! • Just because you have polar bonds, DOESN’T MEAN YOU ARE A POLAR MOLECULE.
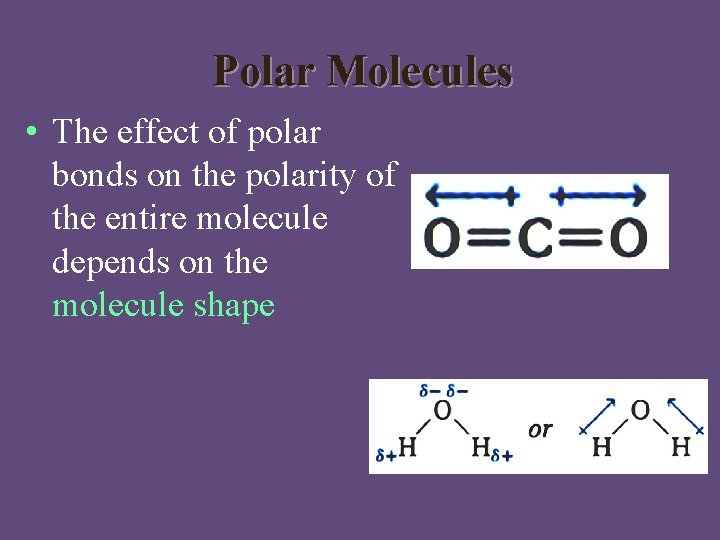
Polar Molecules • The effect of polar bonds on the polarity of the entire molecule depends on the molecule shape
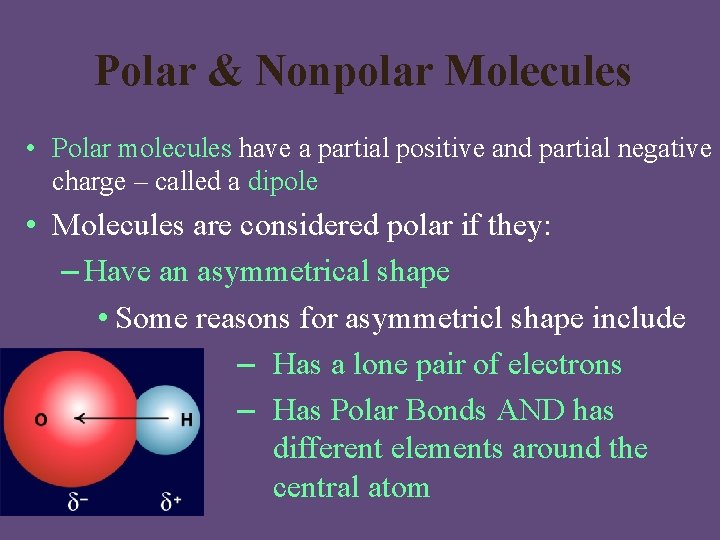
Polar & Nonpolar Molecules • Polar molecules have a partial positive and partial negative charge – called a dipole • Molecules are considered polar if they: – Have an asymmetrical shape • Some reasons for asymmetricl shape include – Has a lone pair of electrons – Has Polar Bonds AND has different elements around the central atom

SYMMETRICAL MOLECULES • This molecule is non-polar due to the symmetrical shape of the molecule but it does contain polar bonds. YOU CAN HAVE A NONPOLAR MOLECULE AND POLAR BONDS!!! Polar bonds H H C H H
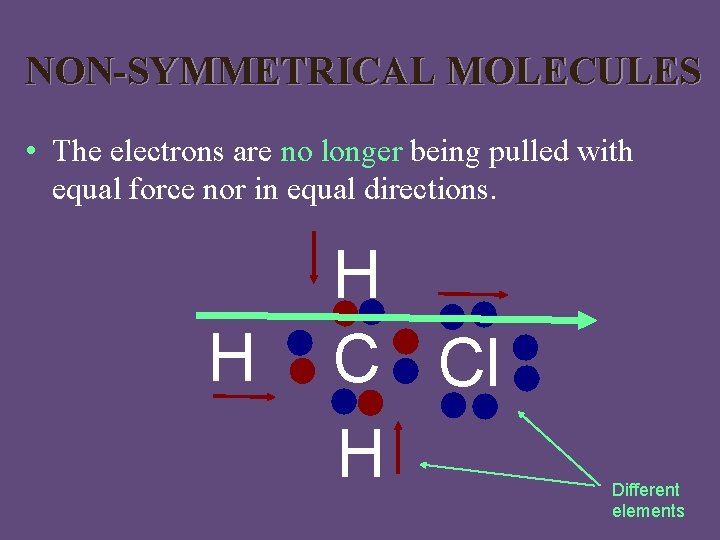
NON-SYMMETRICAL MOLECULES • The electrons are no longer being pulled with equal force nor in equal directions. H H C Cl H Different elements

Polar Molecule Practice • Is this Polar or Non Polar? – Non-Polar + H Cl
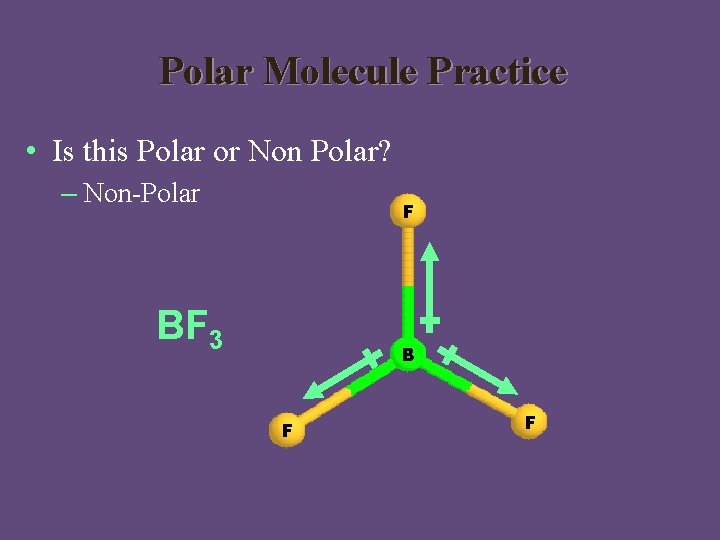
Polar Molecule Practice • Is this Polar or Non Polar? – Non-Polar F BF 3 B F F
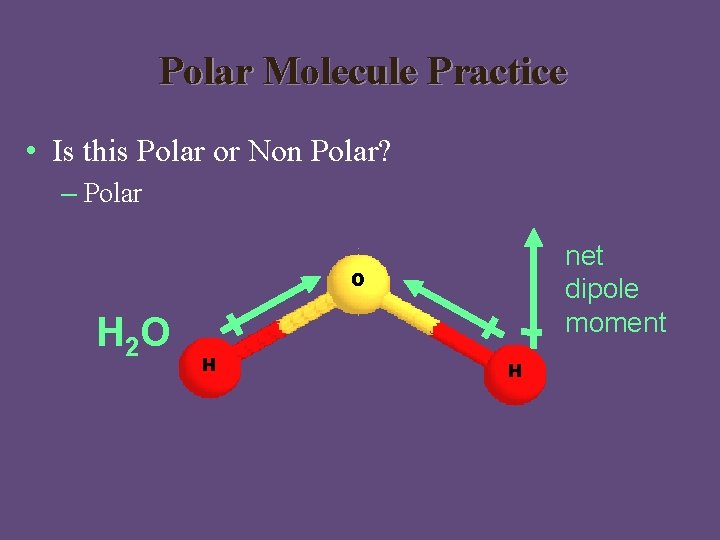
Polar Molecule Practice • Is this Polar or Non Polar? – Polar net dipole moment O H 2 O H H
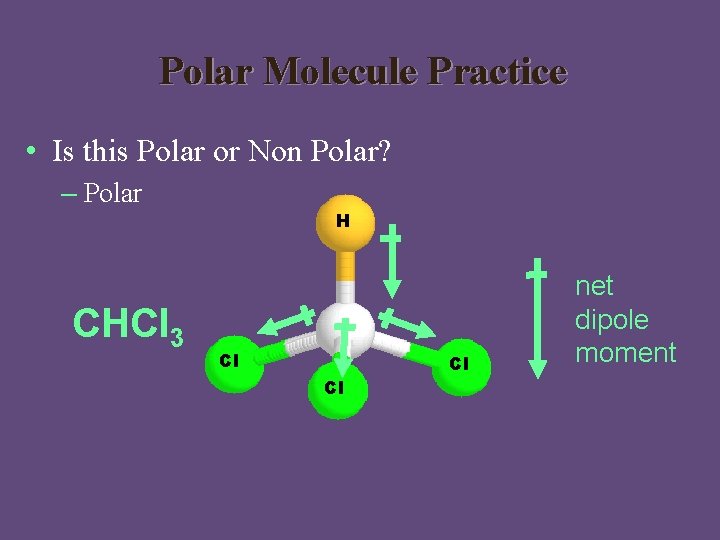
Polar Molecule Practice • Is this Polar or Non Polar? – Polar CHCl 3 H Cl Cl Cl net dipole moment
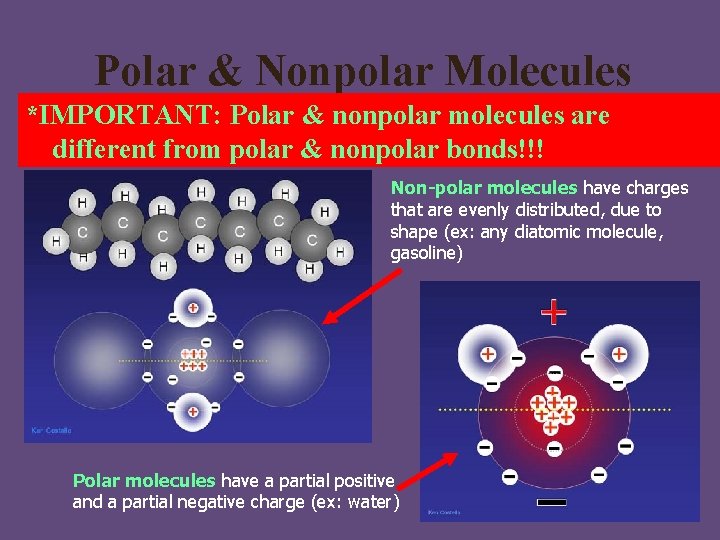
Polar & Nonpolar Molecules *IMPORTANT: Polar & nonpolar molecules are different from polar & nonpolar bonds!!! Non-polar molecules have charges that are evenly distributed, due to shape (ex: any diatomic molecule, gasoline) Polar molecules have a partial positive and a partial negative charge (ex: water)
- Slides: 11