Unit 9 Covalent Bonding Chapters 8 9 Chemistry

Unit 9: Covalent Bonding Chapters 8 & 9 Chemistry 1 K Cypress Creek High School
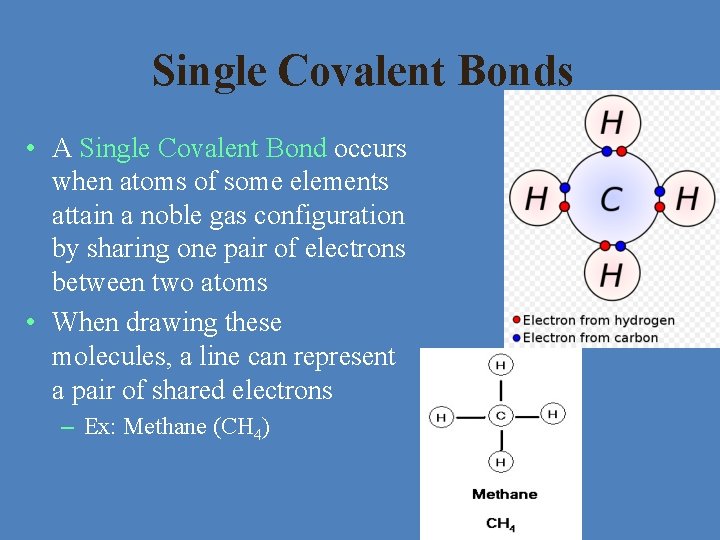
Single Covalent Bonds • A Single Covalent Bond occurs when atoms of some elements attain a noble gas configuration by sharing one pair of electrons between two atoms • When drawing these molecules, a line can represent a pair of shared electrons – Ex: Methane (CH 4)
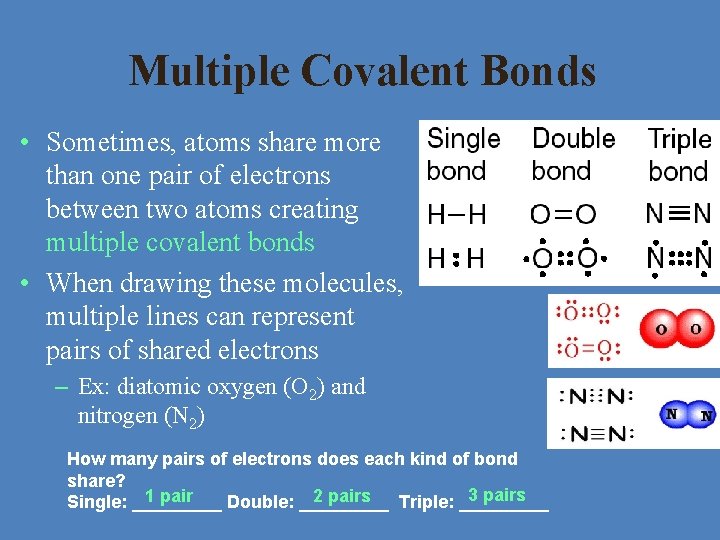
Multiple Covalent Bonds • Sometimes, atoms share more than one pair of electrons between two atoms creating multiple covalent bonds • When drawing these molecules, multiple lines can represent pairs of shared electrons – Ex: diatomic oxygen (O 2) and nitrogen (N 2) How many pairs of electrons does each kind of bond share? 3 pairs 1 pair 2 pairs Single: _____ Double: _____ Triple: _____
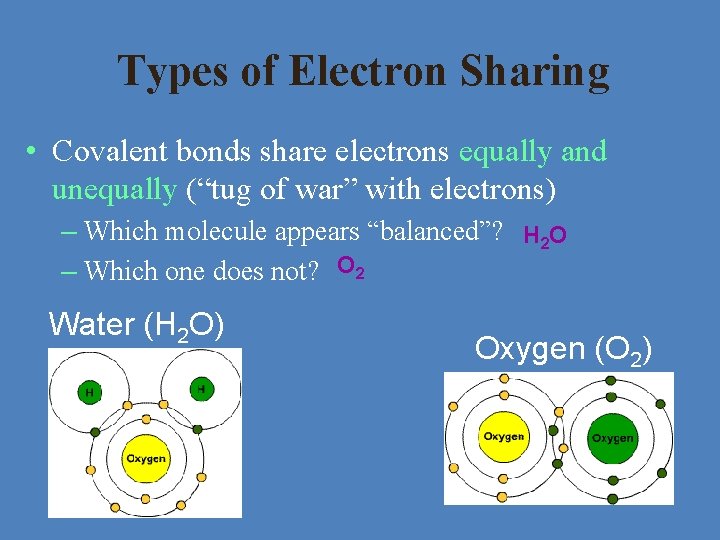
Types of Electron Sharing • Covalent bonds share electrons equally and unequally (“tug of war” with electrons) – Which molecule appears “balanced”? H 2 O – Which one does not? O 2 Water (H 2 O) Oxygen (O 2)
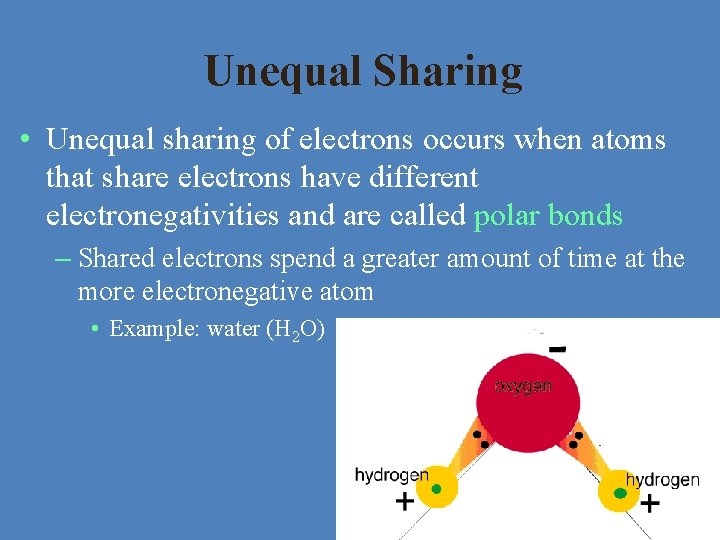
Unequal Sharing • Unequal sharing of electrons occurs when atoms that share electrons have different electronegativities and are called polar bonds – Shared electrons spend a greater amount of time at the more electronegative atom • Example: water (H 2 O)
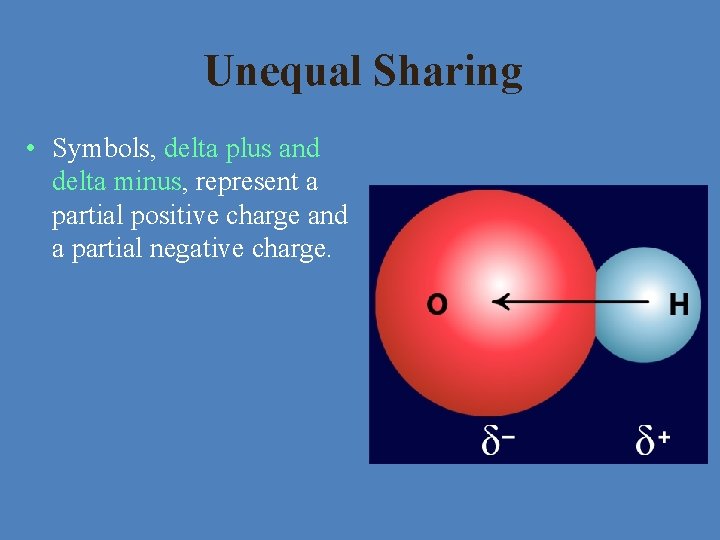
Unequal Sharing • Symbols, delta plus and delta minus, represent a partial positive charge and a partial negative charge.
Unequal Sharing Example: H 2 O • Water Molecule Animation http: //web. jjay. cuny. edu/~acarpi/NSC/5 -bonds. htm
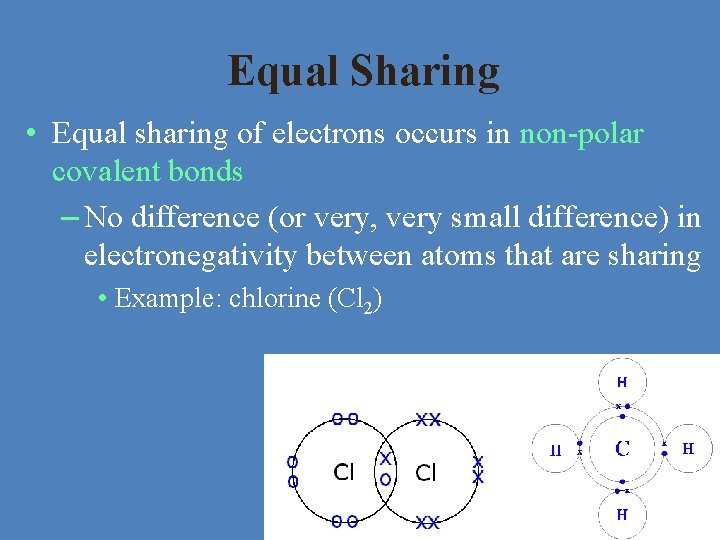
Equal Sharing • Equal sharing of electrons occurs in non-polar covalent bonds – No difference (or very, very small difference) in electronegativity between atoms that are sharing • Example: chlorine (Cl 2)
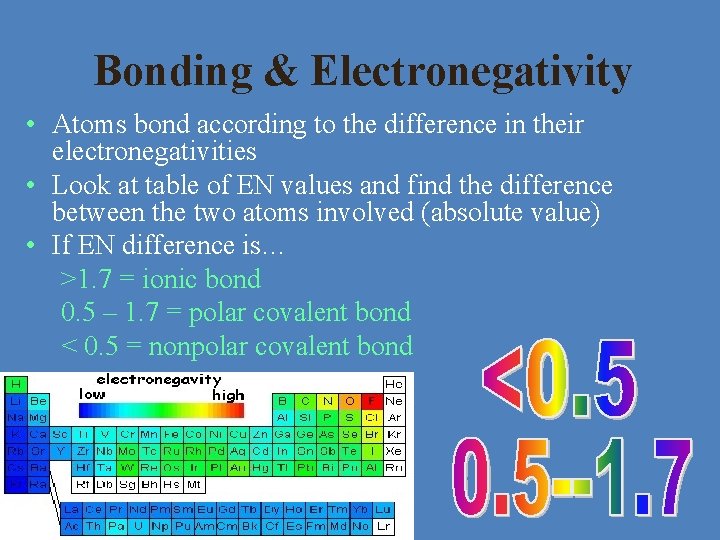
Bonding & Electronegativity • Atoms bond according to the difference in their electronegativities • Look at table of EN values and find the difference between the two atoms involved (absolute value) • If EN difference is… >1. 7 = ionic bond 0. 5 – 1. 7 = polar covalent bond < 0. 5 = nonpolar covalent bond
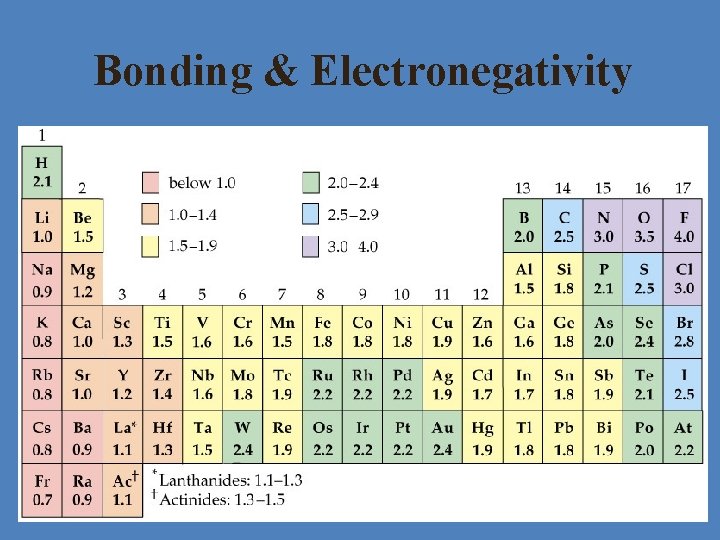
Bonding & Electronegativity
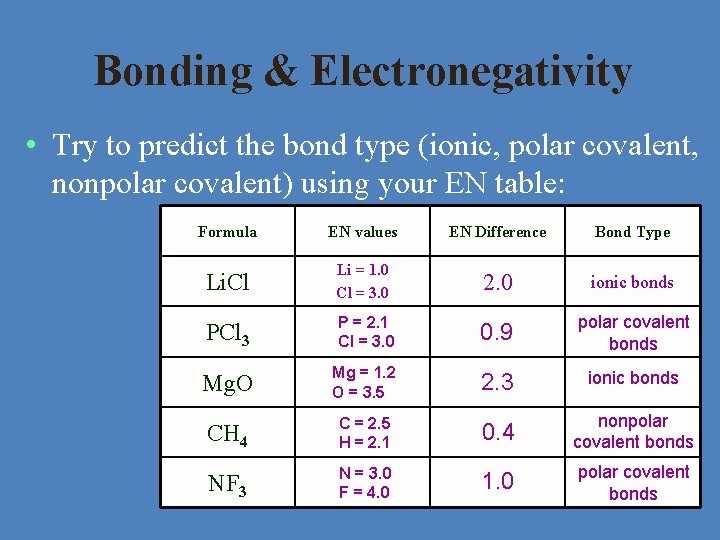
Bonding & Electronegativity • Try to predict the bond type (ionic, polar covalent, nonpolar covalent) using your EN table: Formula EN values EN Difference Bond Type Li. Cl Li = 1. 0 Cl = 3. 0 2. 0 ionic bonds PCl 3 P = 2. 1 Cl = 3. 0 0. 9 polar covalent bonds Mg. O Mg = 1. 2 O = 3. 5 2. 3 ionic bonds CH 4 C = 2. 5 H = 2. 1 0. 4 nonpolar covalent bonds NF 3 N = 3. 0 F = 4. 0 1. 0 polar covalent bonds
- Slides: 11