Unit 9 Chemical Reactions 1 Balancing Chemical Reactions

Unit 9 - Chemical Reactions 1. Balancing Chemical Reactions (Before Break) 2. Types of Chemical Reactions (After Break) 3. Test will be December 9 th 4. Bring latitude tomorrow 5. Get out your notes

Chemical Reactions • Process in which one or more substances are converted into new substances with different physical and chemical properties

• Reactant – substance(s) that enter into a chemical reaction • Product – substance(s) that is produced by a chemical reaction

• A chemical reaction occurs when reactants change into products Reactants Products

• Reasons for Reactions • Atoms exchanging or sharing electrons to obtain full set of valence electrons • New substances are produced as existing bonds are broken, atoms rearranged, and new bonds formed • Transfer of energy

Chemical Equations • Like Math • Word equation problems • Formula equation problems

• Word equation example • Calcium reacts with oxygen to produce ( ) calcium oxide
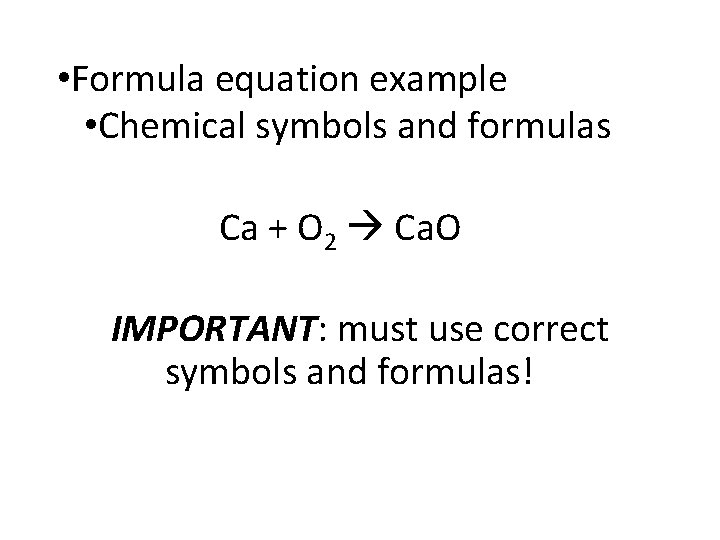
• Formula equation example • Chemical symbols and formulas Ca + O 2 Ca. O IMPORTANT: must use correct symbols and formulas!
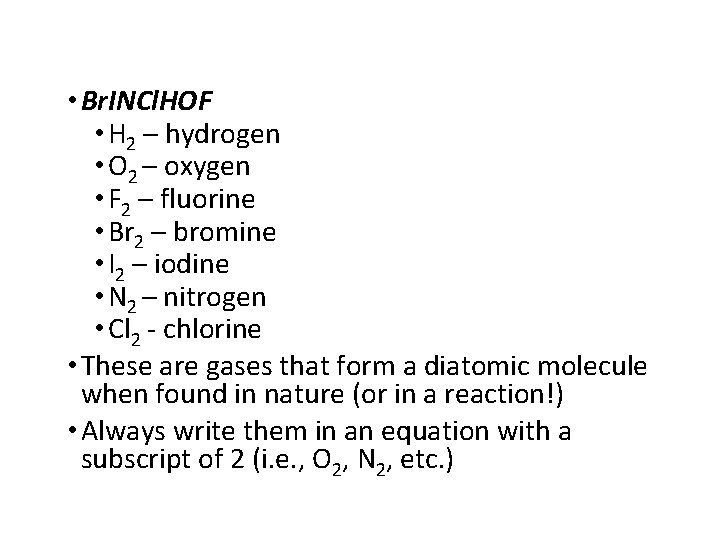
• Br. INCl. HOF • H 2 – hydrogen • O 2 – oxygen • F 2 – fluorine • Br 2 – bromine • I 2 – iodine • N 2 – nitrogen • Cl 2 - chlorine • These are gases that form a diatomic molecule when found in nature (or in a reaction!) • Always write them in an equation with a subscript of 2 (i. e. , O 2, N 2, etc. )

• To write a complete reaction, states of matter must be included for reactants and products • Solid (s) • Liquid (l) • Gas (g) • If dissolved in water, Aqueous (aq)

Symbols Used in Chemical Equations Yields: indicates result of reaction Reversible reaction Indicates a precipitate (solid) Indicates a gaseous product (s) (l) (g) (aq) Pt Reactant or product in a solid state Reactant or product in a liquid state Reactant or product in a gaseous state Reactant or product in a aqueous state Reactants are heated Presence of a catalyst

• Balancing Chemical Equations • Remember: law of conservation of matter must be obeyed! • The number of atoms of each element must be the same before and after the reaction

• Coefficients • Whole numbers written before the formulas for reactants and products • They are used to balance the equation • Example: • 2 X, where 2 is the coefficient

• Subscripts are NEVER changed in the formula for reactants or products • These are set by the charges on the ions or the number of atoms in molecule

• Methane reacts with oxygen to produce carbon dioxide and water CH 4 + O 2 CO 2 + H 2 O List # of each atom • 1 C 1 C • 4 H 2 H • 2 O 3 O • How can you make them equal, by using only coefficients?

Steps to Balance an Equation • Step 1: List all the elements in the equation underneath the center of the equation • Step 2: Count how many of each element is on the reactant side then the product side • Step 3: Is this balanced or unbalanced? • Step 4: Balance the equation if necessary by adding coefficients only. Never change subscripts!!!
- Slides: 16