Unit 9 Atomic Theory and Periodicity Section 2



- Slides: 11

Unit 9: Atomic Theory and Periodicity Section 2: Quantum Mechanical Theory

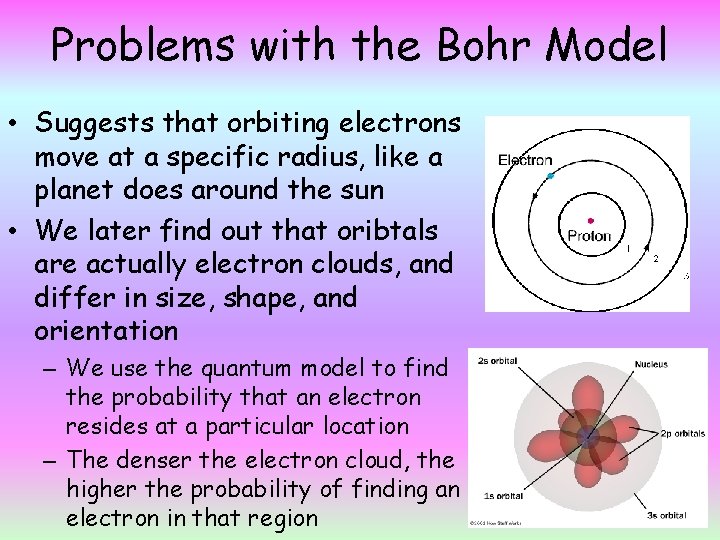
Problems with the Bohr Model • Suggests that orbiting electrons move at a specific radius, like a planet does around the sun • We later find out that oribtals are actually electron clouds, and differ in size, shape, and orientation – We use the quantum model to find the probability that an electron resides at a particular location – The denser the electron cloud, the higher the probability of finding an electron in that region

Quantum Numbers • Specifies the properties of atomic orbitals and the properties of electrons in orbitals • Each electron can be assigned a set of four quantum numbers – These numbers are the electron’s address

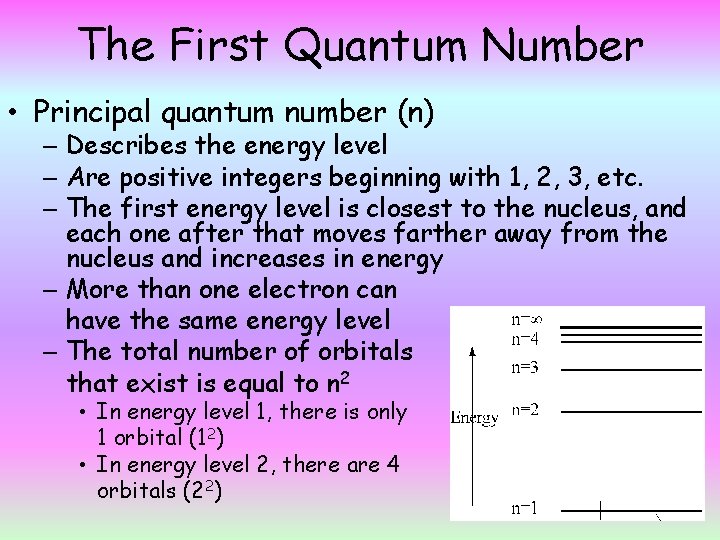
The First Quantum Number • Principal quantum number (n) – Describes the energy level – Are positive integers beginning with 1, 2, 3, etc. – The first energy level is closest to the nucleus, and each one after that moves farther away from the nucleus and increases in energy – More than one electron can have the same energy level – The total number of orbitals that exist is equal to n 2 • In energy level 1, there is only 1 orbital (12) • In energy level 2, there are 4 orbitals (22)

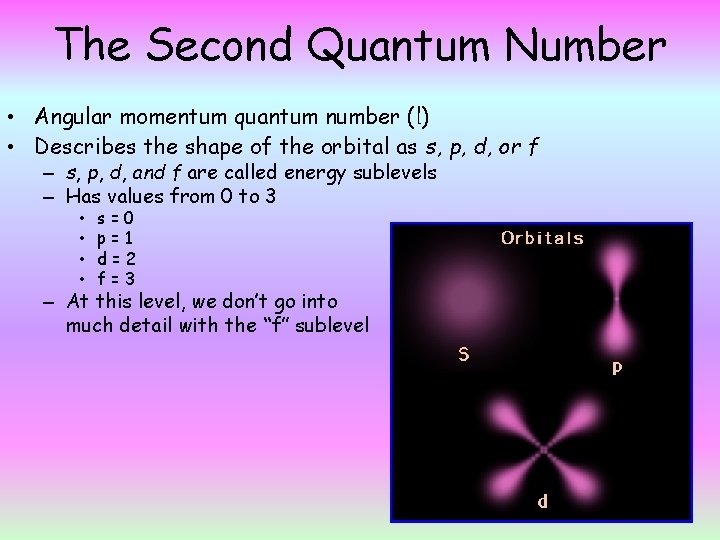
The Second Quantum Number • Angular momentum quantum number (l) • Describes the shape of the orbital as s, p, d, or f – s, p, d, and f are called energy sublevels – Has values from 0 to 3 • • s=0 p=1 d=2 f=3 – At this level, we don’t go into much detail with the “f” sublevel

The Second Quantum Number • s sublevel has one orbital, an s orbital – A capacity of two electrons – Makes up columns 1 and 2 on the p. t. • p sublevel has three orbitals, x, y, and z – A capacity of six electrons – Makes up columns 13 to 18 on the p. t. • d sublevel has five orbitals – A capacity of ten electrons – Makes up columns 3 to 12 on the p. t. • f sublevel has seven orbitals – A capacity of 14 electrons – Makes up the lanthanides and actinides on the p. t.

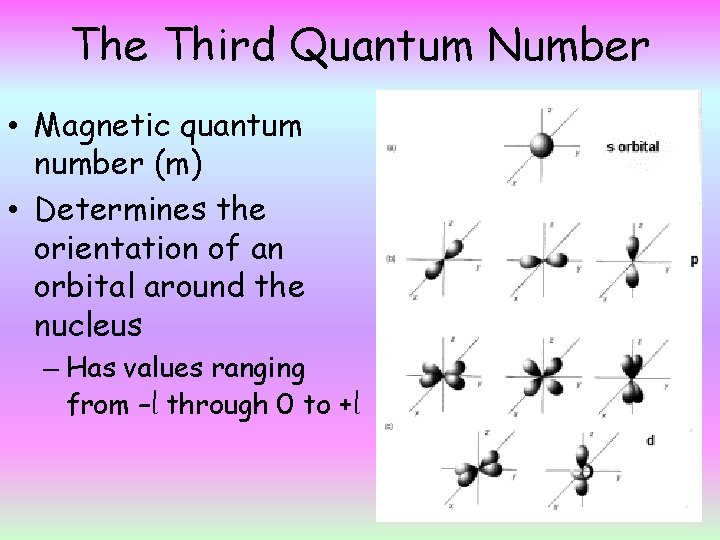
The Third Quantum Number • Magnetic quantum number (m) • Determines the orientation of an orbital around the nucleus – Has values ranging from –l through 0 to +l

The Fourth Quantum Number • Spin quantum number – Electrons are said to have either a +½ spin or a -½ spin • Also known as a clockwise and counterclockwise spin – Within an orbital, the first electron has a positive spin and the second electron has a negative spin • A single orbital can hold a maximum of two electrons, which must have opposite spin states

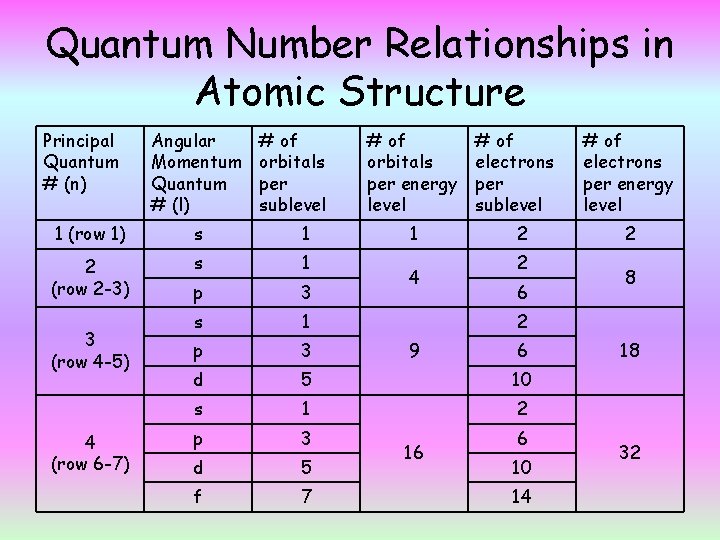
Quantum Number Relationships in Atomic Structure Principal Quantum # (n) Angular Momentum Quantum # (l) # of orbitals per sublevel # of orbitals per energy level # of electrons per sublevel 1 (row 1) s 1 2 (row 2 -3) s 1 p 3 d 5 10 s 1 2 p 3 d 5 f 7 3 (row 4 -5) 4 (row 6 -7) 1 4 2 2 6 # of electrons per energy level 2 8 2 9 16 6 6 10 14 18 32

Electron Configuration • A way of describing each of an element’s electrons • Written using the following steps: – Find out the # of electrons for the element – Start with the 1 s part • 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f, etc. – For each orbital, insert the maximum # of electrons in the exponent position – Continue until each element’s electrons has been described – The superscripts should add up to the element’s atomic # (indicates # of electrons)

Electron Configuration Examples • The element sodium has 11 electrons – 1 s 22 p 63 s 1 • The sum of the superscript #s equal 11 – 2+2+6+1 = 11 • The coefficients indicate the energy level and row # on the p. t. • The alphabet’s superscripts indicate the location and column # on the p. t. • The element silver has 47 electrons – 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 9