Unit 8 The Periodic Table Trends Notes goals
Unit 8: The Periodic Table Trends

Notes goals • Understand basic history and arrangement of periodic table • Be able to use the periodic table to predict chemical trends • Be able to use periodic trends to determine the identity of elements

History • Dmitri Mendeleev (1869, Russian) – Organized elements by increasing atomic mass. – Elements with similar properties were grouped together. – There were some discrepancies.

History • Henry Mosely (1913, British) – Organized elements by increasing atomic number. – Resolved discrepancies in Mendeleev’s arrangement.

Arrangement • Period = a single row going across left to right – Numbered from 1 to 7 • Group/Family = a column going down top to bottom – Different numbering systems (1 -18) or (1 A – 8 A, etc. ) – Elements in the same group have similar physical and chemical characteristics

Periodic Trends • • Effective Nuclear Charge Atomic Radius Ionization Energy Electron affinity Electronegativity Overall Reactivity

Effective Nuclear Charge • Valence electrons = electrons in outer level • Core electrons = electrons in levels underneath outer level • Valence electrons are: – Attracted to protons in nucleus – Repelled by core electrons which are in the way (shielding)

Effective Nuclear Charge • Effective Nuclear Charge = the net attraction of the valence electrons to the nucleus – Greater effective nuclear charge means valence electrons are closer to the nucleus
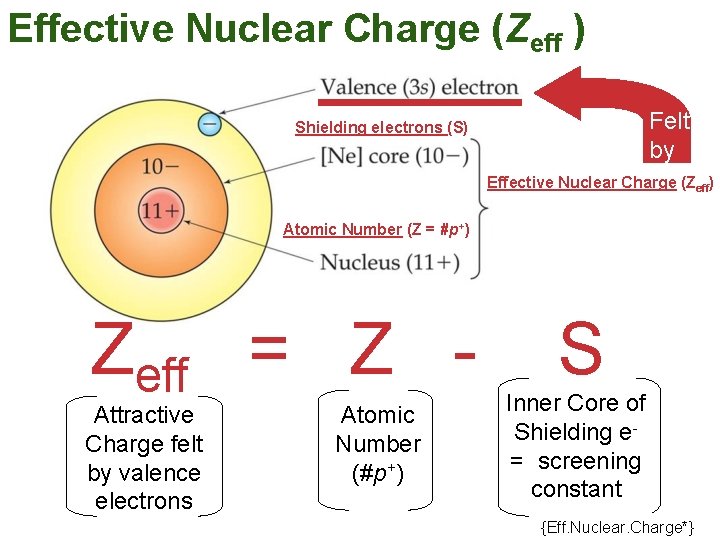
Effective Nuclear Charge (Zeff ) Felt by Shielding electrons (S) Effective Nuclear Charge (Zeff) Atomic Number (Z = #p+) Zeff = Z - Attractive Charge felt by valence electrons Atomic Number (#p+) S Inner Core of Shielding e= screening constant {Eff. Nuclear. Charge*}
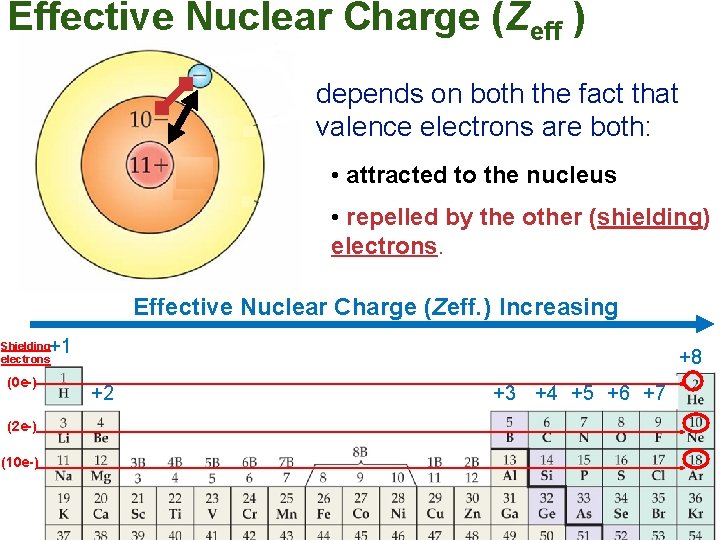
Effective Nuclear Charge (Zeff ) depends on both the fact that valence electrons are both: • attracted to the nucleus • repelled by the other (shielding) electrons. Effective Nuclear Charge (Zeff. ) Increasing +1 Shielding electrons (0 e-) (2 e-) (10 e-) +8 +2 +3 +4 +5 +6 +7
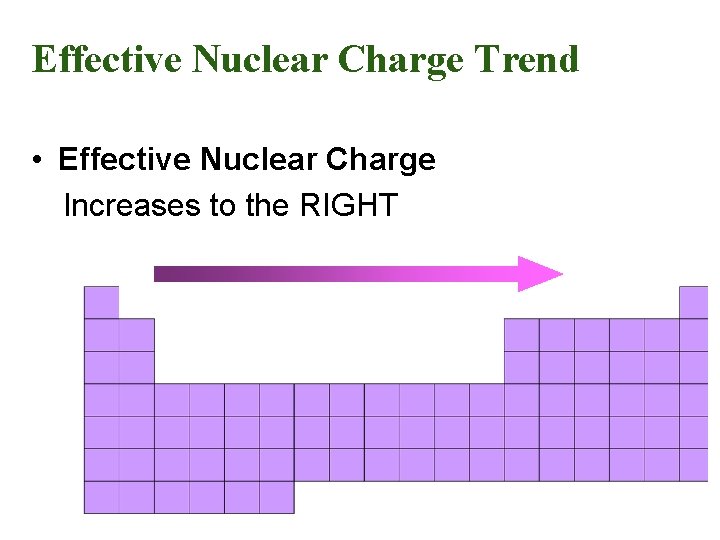
Effective Nuclear Charge Trend • Effective Nuclear Charge Increases to the RIGHT
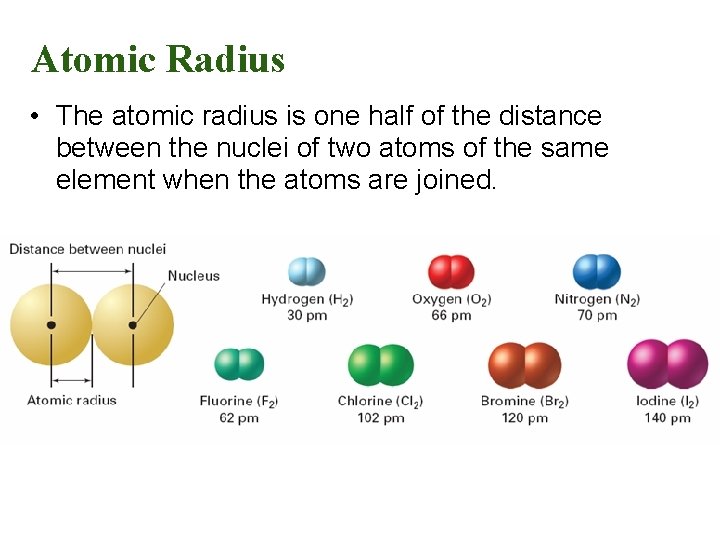
6. 3 Atomic Radius • The atomic radius is one half of the distance between the nuclei of two atoms of the same element when the atoms are joined.

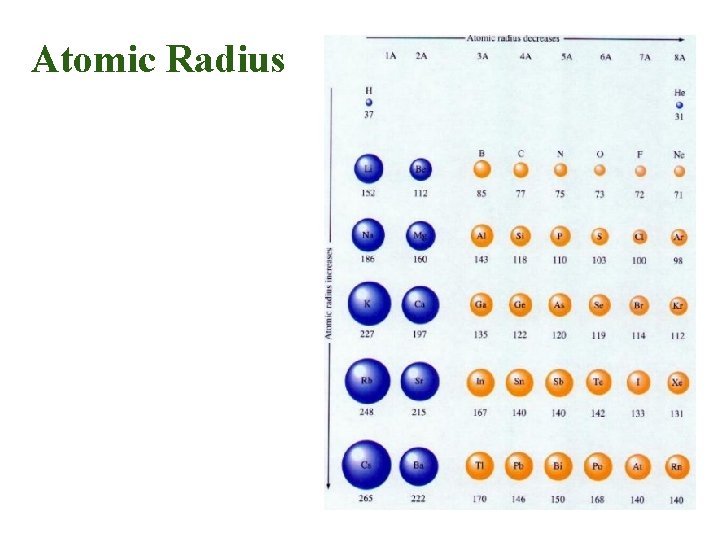
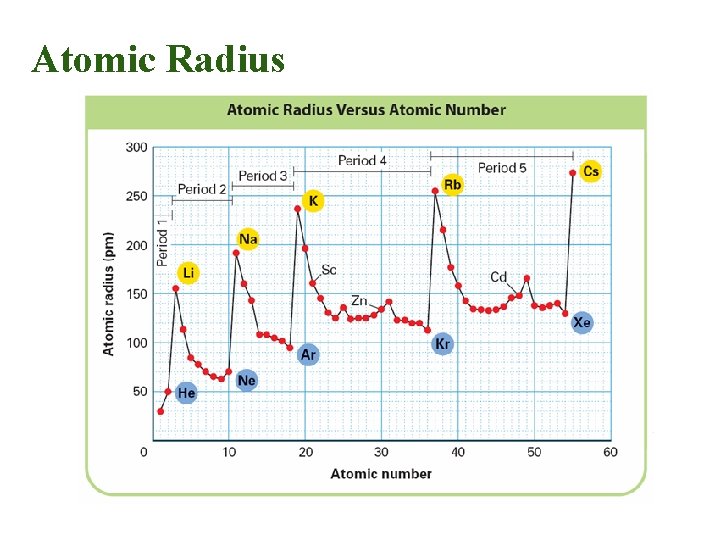
Atomic Radius • Why larger going down? – Higher energy levels have larger orbitals • Why smaller to the right? – Increased nuclear charge without additional shielding pulls electrons in tighter
Atomic Radius
Atomic Radius
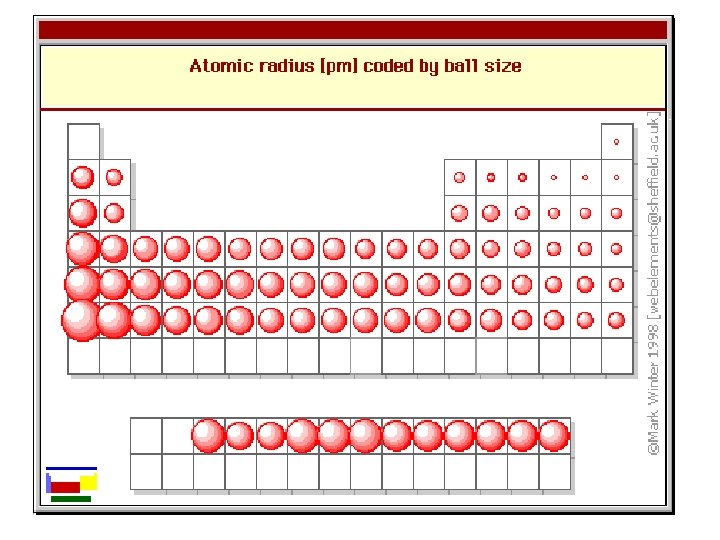
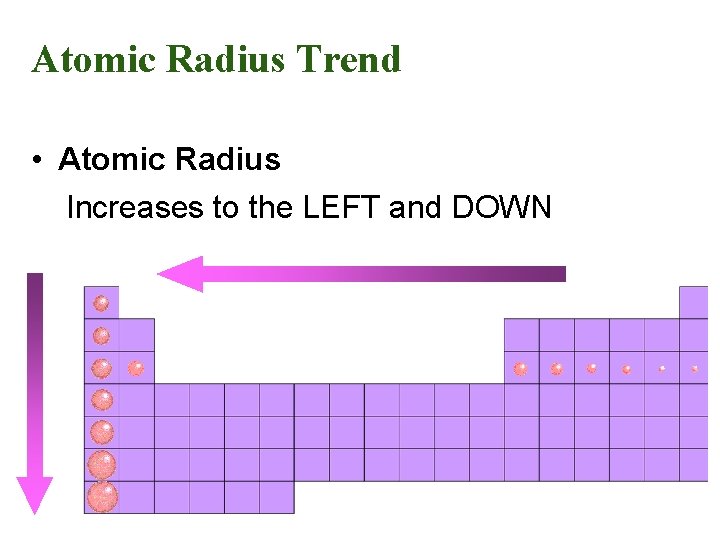
Atomic Radius Trend • Atomic Radius Increases to the LEFT and DOWN
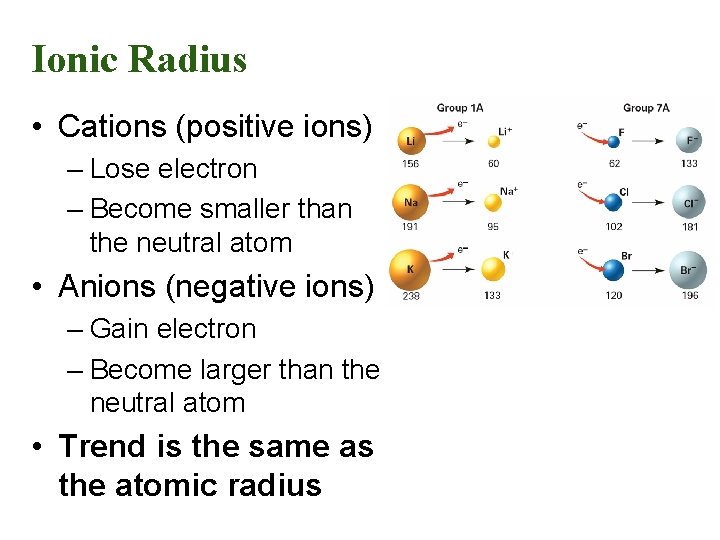
Ionic Radius • Cations (positive ions) – Lose electron – Become smaller than the neutral atom • Anions (negative ions) – Gain electron – Become larger than the neutral atom • Trend is the same as the atomic radius

Summary of Ionic Radius • Cations are always smaller than the original atom. • The entire outer EL is removed during ionization. • Conversely, anions are always larger than the original atom. • Electrons are added to the outer EL.
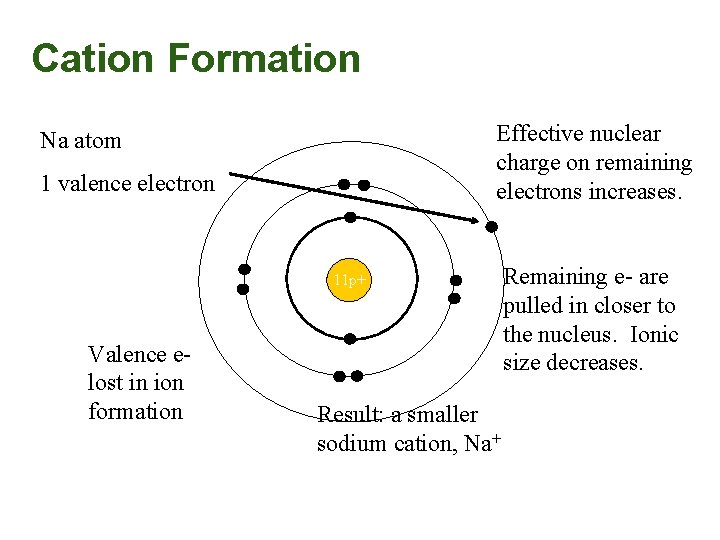
Cation Formation Effective nuclear charge on remaining electrons increases. Na atom 1 valence electron 11 p+ Valence elost in ion formation Result: a smaller sodium cation, Na+ Remaining e- are pulled in closer to the nucleus. Ionic size decreases.
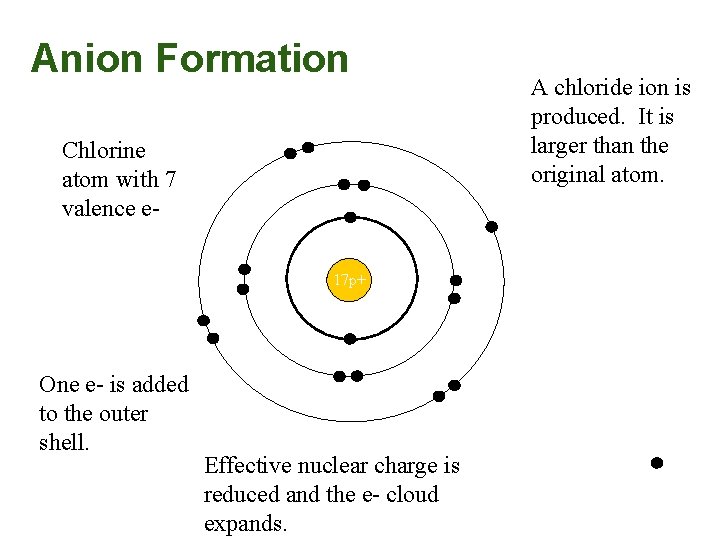
Anion Formation Chlorine atom with 7 valence e 17 p+ One e- is added to the outer shell. Effective nuclear charge is reduced and the e- cloud expands. A chloride ion is produced. It is larger than the original atom.
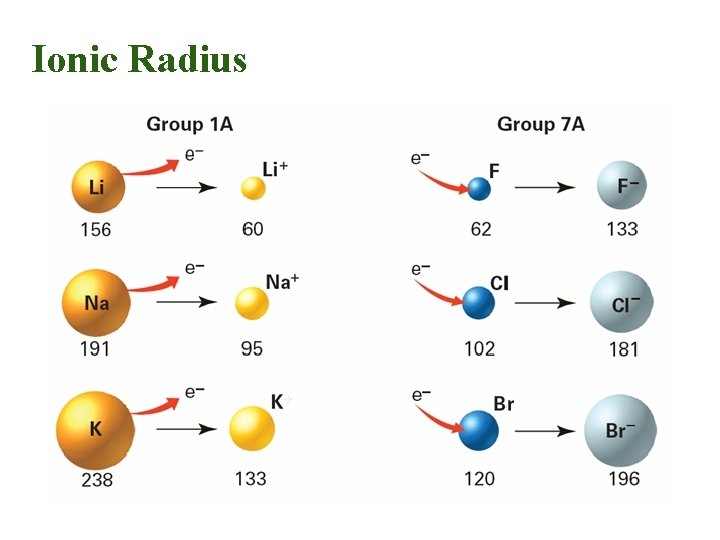
Ionic Radius
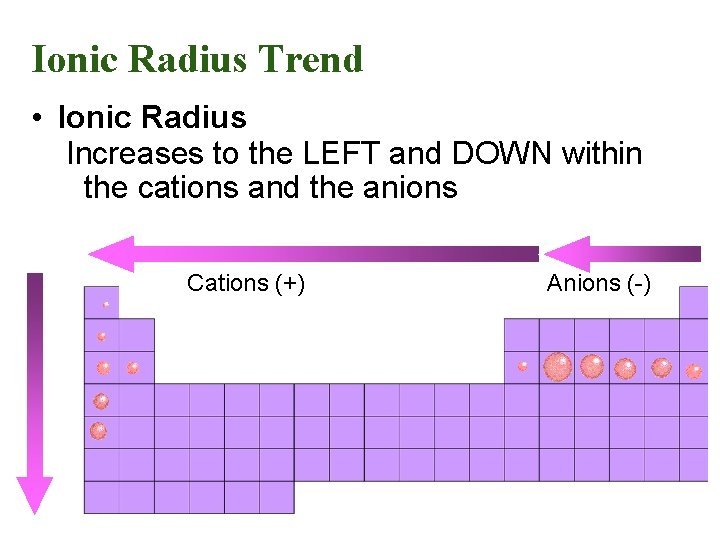
Ionic Radius Trend • Ionic Radius Increases to the LEFT and DOWN within the cations and the anions Cations (+) Anions (-)

Ionization Energy • Ionization Energy = Energy required to remove an electron from a neutral atom • Smaller atoms have electrons closer to the nucleus so they are held tighter. – This means a higher ionization energy is required to take an electron away. • There a few exceptions where s, p, or d orbitals are filled or half-filled which creates a more stable structure
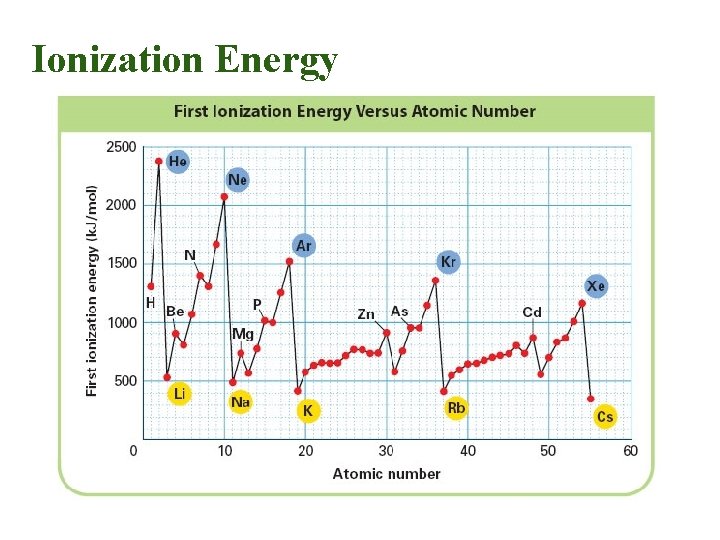
Ionization Energy
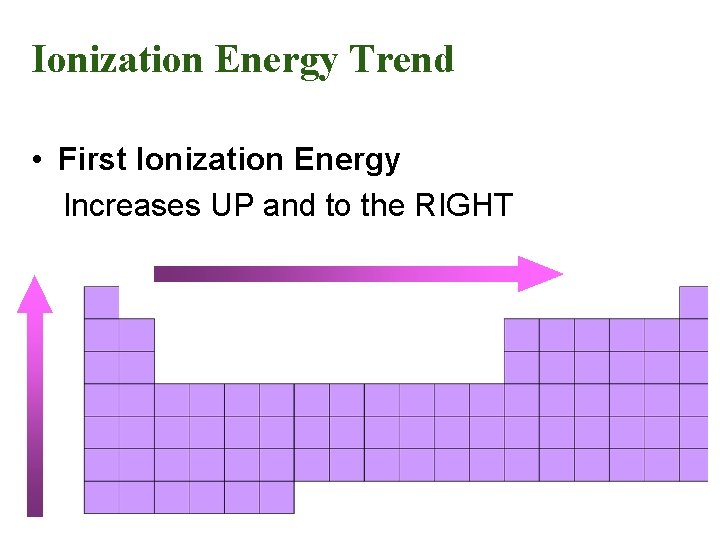
Ionization Energy Trend • First Ionization Energy Increases UP and to the RIGHT

Electron Affinity • What does the word ‘affinity’ mean? • Electron affinity is the energy change that occurs when an atom gains an electron (also measured in k. J). • Where ionization energy is always endothermic, electron affinity is usually exothermic, but not always.
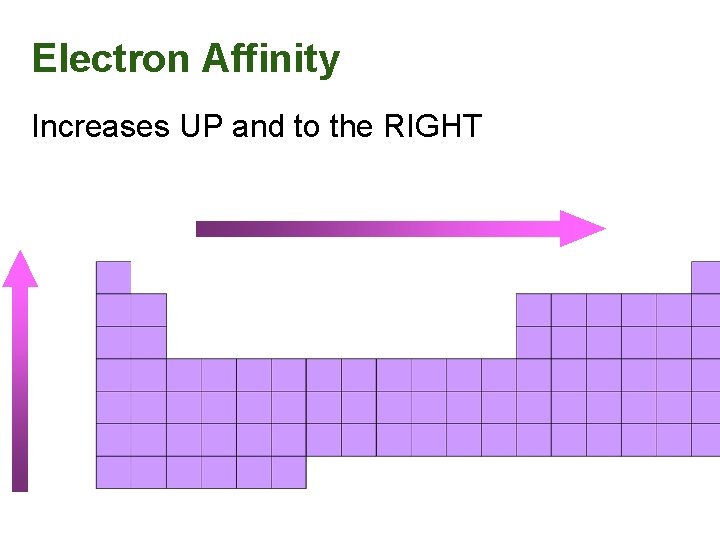
Electron Affinity Increases UP and to the RIGHT

Electronegativity • Electronegativity is a measure of an atom’s attraction for another atom’s electrons. • ranges from 0 to 4. • Generally, metals are electron givers and have low electronegativities. • Nonmetals are electron takers and have high electronegativities. • What about the noble gases?
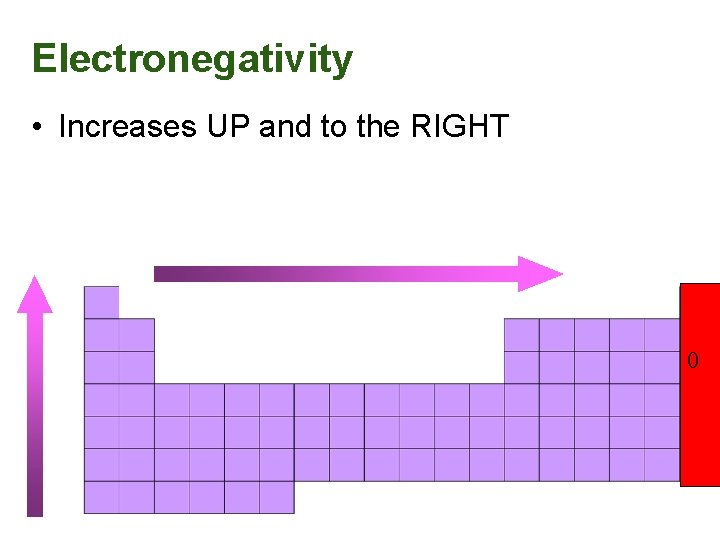
Electronegativity • Increases UP and to the RIGHT 0

Overall Reactivity • This ties all the previous trends together in one package. • However, we must treat metals and nonmetals separately. • The most reactive metals are the largest since they are the best electron givers. • The most reactive nonmetals are the smallest ones, the best electron takers.
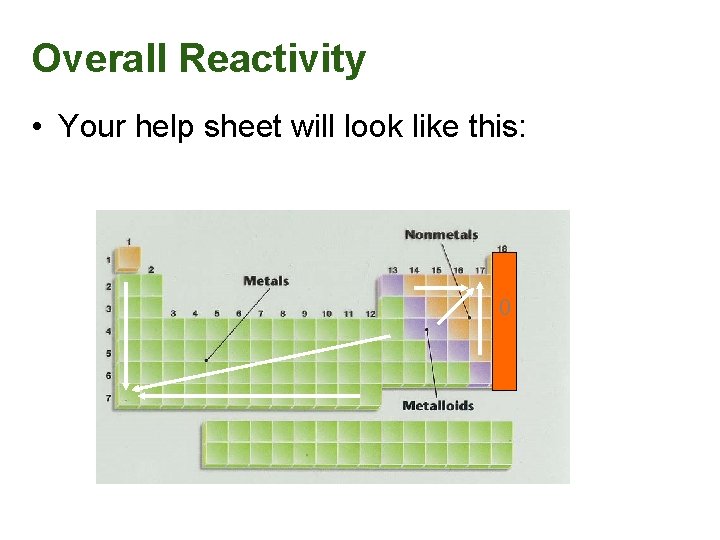
Overall Reactivity • Your help sheet will look like this: 0

The Octet Rule • The “goal” of most atoms (except H, Li and Be) is to have an octet or group of 8 electrons in their valence energy level. • They may accomplish this by either giving electrons away or taking them. • Metals generally give electrons, nonmetals take them from other atoms. • Atoms that have gained or lost electrons are called ions.
- Slides: 33