Unit 8 Temperature and Matter I Self Learning








- Slides: 25

Unit 8: Temperature and Matter I Self Learning Package Click here to proceed to next page

Topics to be covered 1. Macroscopic Properties of Solids, Liquids and Gases 2. Brownian Motion 3. Kinetic Model 1. Changing of State 2. Heating and Cooling Curves 3. Evaporation 4. Internal Energy

Textbook References • Marshall Cavendish Textbook: – Chapter 9 (Pg 153 -164) – Chapter 11 (Pg 192 -197; 202 -204) • GLM Red Book – Unit 8 (Pg 131 -142) – Unit 11 (Pg 171; 182 -189)

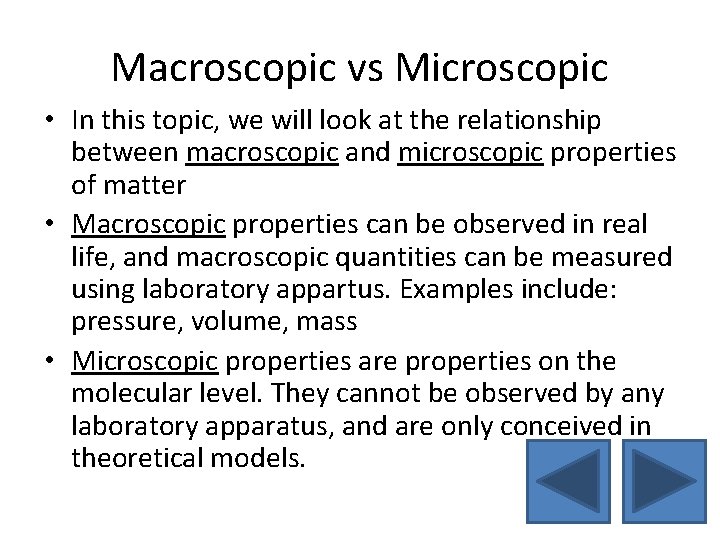
Macroscopic vs Microscopic • In this topic, we will look at the relationship between macroscopic and microscopic properties of matter • Macroscopic properties can be observed in real life, and macroscopic quantities can be measured using laboratory appartus. Examples include: pressure, volume, mass • Microscopic properties are properties on the molecular level. They cannot be observed by any laboratory apparatus, and are only conceived in theoretical models.

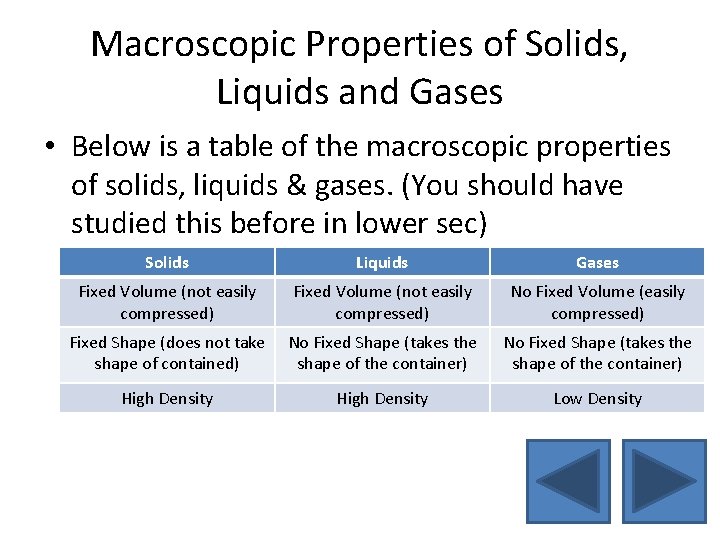
Macroscopic Properties of Solids, Liquids and Gases • Below is a table of the macroscopic properties of solids, liquids & gases. (You should have studied this before in lower sec) Solids Liquids Gases Fixed Volume (not easily compressed) No Fixed Volume (easily compressed) Fixed Shape (does not take shape of contained) No Fixed Shape (takes the shape of the container) High Density Low Density

Brownian Motion • Named after Robert Brown (the scientist which first observed this) Brownian motion is the experiment which provides evidence for the kinetic model of matter. • Read Marshall Cavendish textbook pg 157. You need to know – setup of the experiment – what is observed – conclusion of experiment

Brownian Motion • In the Brownian Motion experiment, smoke particles are seen to be moving randomly • The explanation for this is that they are constantly being bombarded by air molecules, which are also moving randomly • IMPORTANT: air molecules themselves are not directly observed. Only the effects of air molecules on the smoke particles.

Kinetic Model of Matter • Also known as the “Particulate Model of Matter” • The Kinetic Model is a scientific model postulated to explain the observations in the Brownian Motion experiment, as well as the different macroscopic properties of solids, liquids and gases • (Side Note: the Kinetic Model is only a preliminary model. It has since been updated with more advanced and comprehensive models. )

Kinetic Model of Matter • Key Concepts: • 1) All matter is made up of tiny particles • 2) Forces of attraction exist between these particles • 3) The higher the temperature, the faster the movement of the particles (i. e. the greater the Kinetic Energy)

Kinetic Model of Matter • In Solids: • Particles are closely packed together, in a regular pattern • Particles are held in position by strong attractive forces between them • This explains why solids have high density, fixed shape and fixed volume • Particles vibrate about fixed positions. The higher the temperature, the more vigorous the vibrations

Kinetic Model of Matter • In Liquids: • Particles are randomly arranged, but still quite closely packed together • Particles are free to move about within the liquid • This explains why liquids have high density, fixed volume, but no fix shape • When heated up, particles move about faster

Kinetic Model of Matter • In Gases: • Particles are very far apart from each other • Particles move about randomly and at high speed • This explains why gases have low densities, no fixed volume or shape • When heated up, particles move about even faster

Try this applet! • Click on the link below to try an applet which shows you the kinetic model of matter in solids, liquids and gases • https: //phet. colorado. edu/en/simulation/stat es-of-matter-basics

Changing of State • The process of changing from liquid to gas is called boiling • The process of changing from solid to liquid is called melting • During boiling and melting, thermal energy is absorbed, and is used to overcome forces of attraction between the particles • The average speed of the particles do not increase, which is why there is no increase in temperature during boiling or melting

Changing of State • The process of changing from gas to liquid is called condensation • The process of changing from liquid to solid is called solidification (or freezing) • During freezing or condensation, thermal energy is released when the particles get attracted by the forces of attraction between them • The average speed of particles do not decrease, which is why there is no change of temperature during condensation or freezing.

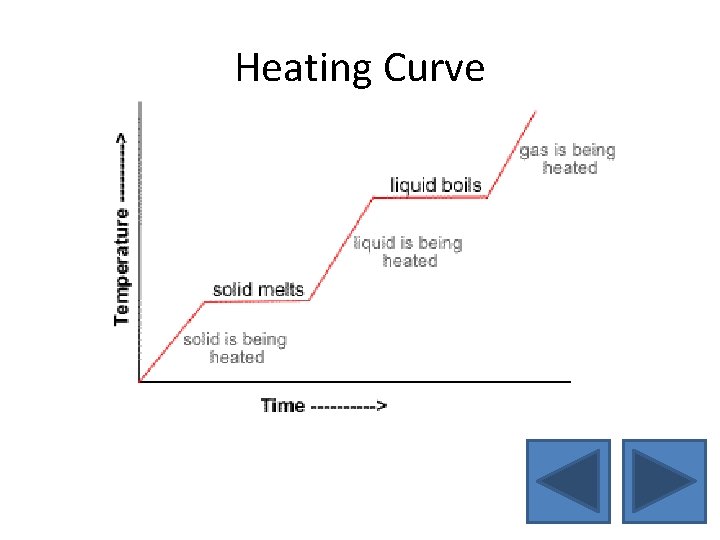
Heating Curve • When an object is constantly being heated, the graph of its temperature against time is called a heating curve • The heating curve is flat during melting and boiling because there is no increase in temperature

Heating Curve

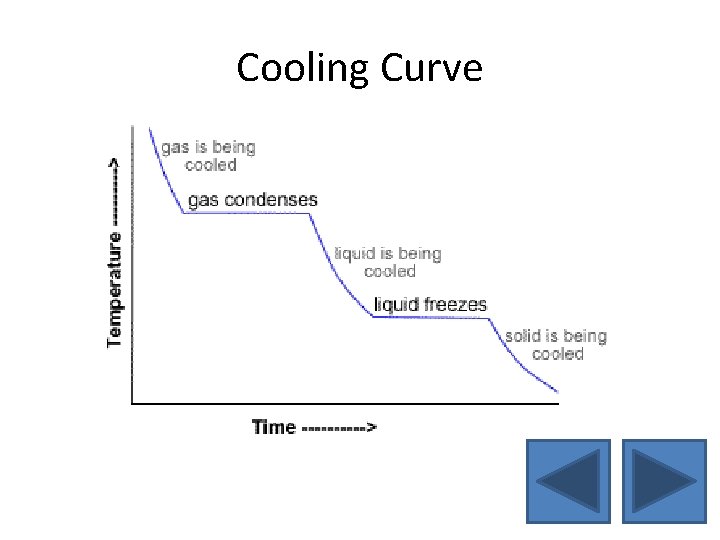
Cooling Curve • When an object has thermal energy constantly being released, the graph of its temperature against time is called a cooling curve • The cooling curve is flat during condensation and freezing because there is no decrease in temperature

Cooling Curve

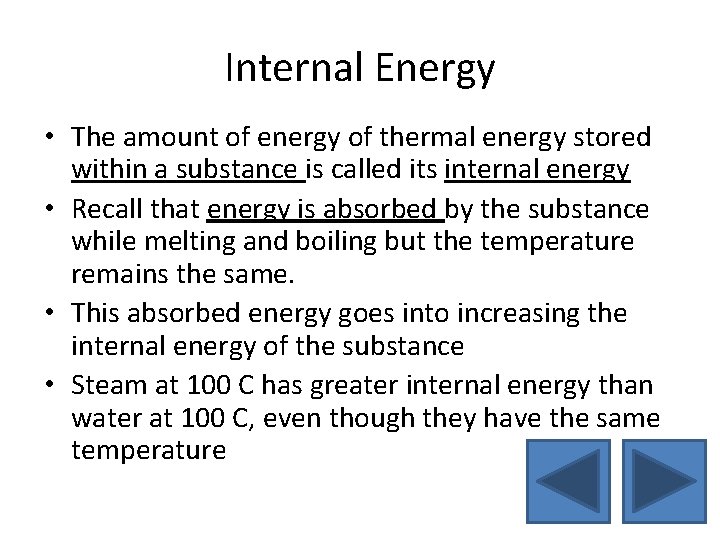
Internal Energy • The amount of energy of thermal energy stored within a substance is called its internal energy • Recall that energy is absorbed by the substance while melting and boiling but the temperature remains the same. • This absorbed energy goes into increasing the internal energy of the substance • Steam at 100 C has greater internal energy than water at 100 C, even though they have the same temperature

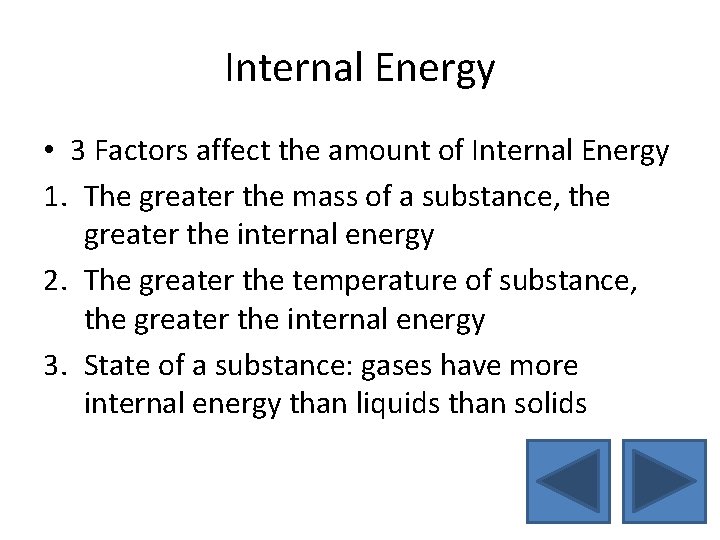
Internal Energy • 3 Factors affect the amount of Internal Energy 1. The greater the mass of a substance, the greater the internal energy 2. The greater the temperature of substance, the greater the internal energy 3. State of a substance: gases have more internal energy than liquids than solids

Evaporation • Evaporation is the process where a liquid converts into gas below the boiling point • Unlike boiling, evaporation can happen at any temperature • Unlike boiling, evaporation only occurs at the surface of the liquid • Evaporation causes as “cooling effect”, lowering the temperature of the liquid

Evaporation • Recall that temperature of a substance is related to the average speed of its particles • In any liquid, it has a mixture of faster and slower moving particles • At the surface of the liquid, the faster moving particles break free from the liquid and escape into the atmosphere • Leaving behind the slower particles. Since average speed of particles is now lower, the temperature of the liquid is now lower

Summary 1. Macroscopic Properties of Solids, Liquids and Gases 2. Brownian Motion 3. Kinetic Model 1. Changing of State 2. Heating and Cooling Curves 3. Evaporation 4. Internal Energy

Assignment • Click on the link below to complete Part 1 of your June holiday assignment. Note there is also a Part 2 (Unit 9). • https: //docs. google. com/a/preshigh. edu. sg/form s/d/1 Ni 24 T 2 h_Y 8 f 4 jyfu. JWIM 4 V_EPy. Ksu. SC 7 BUzy Tn. Lg. V 8 g/viewform? usp=send_form • Note: you need to login to your preshigh account • Due date: 9 July 11: 59 pm • (Press “Esc” to end this presentation)