Unit 8 Kinetics Equilibrium Kinetics Chemical Kinetics The

Unit 8 Kinetics & Equilibrium

Kinetics Chemical Kinetics: The mechanics of a reaction – how it actually occurs physically.
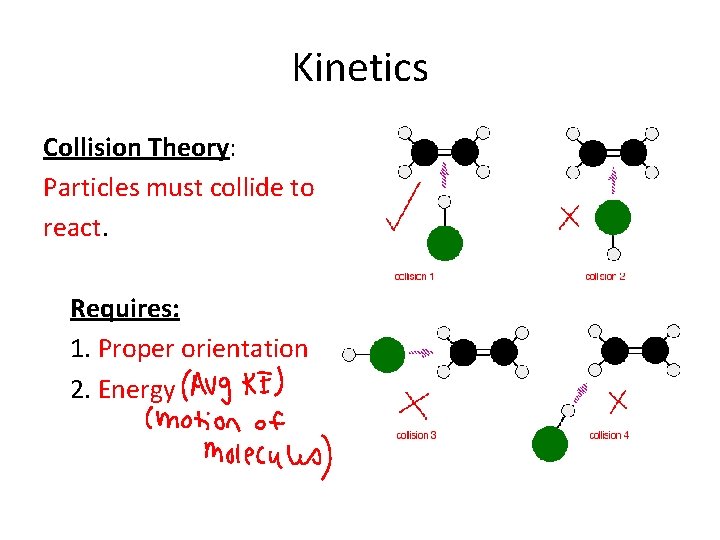
Kinetics Collision Theory: Particles must collide to react. Requires: 1. Proper orientation 2. Energy

Collision Theory Animation: http: //drmackay. org/kinetics/collision/2 collision A_ana. html

Kinetics • The collision theory says that the more collisions in a system, the more likely combinations of molecules will happen. • If there a higher number of collisions in a system, more combinations of molecules will occur. The reaction will go faster, and the rate of that reaction will be higher.
![Kinetics Rate of Reaction: How fast a reaction occurs. [reactants] [products] time Kinetics Rate of Reaction: How fast a reaction occurs. [reactants] [products] time](http://slidetodoc.com/presentation_image_h2/ad601efc68e96f47a9851e885771b90e/image-6.jpg)
Kinetics Rate of Reaction: How fast a reaction occurs. [reactants] [products] time

Collision Theory https: //www. youtube. com/watch? v=Ott. RV 5 yk. P 7 A

Decrease in temp = particles move slower = less collisions w/ less energy rxn rate decreases Factor Increase Temperature Increase temp = particles move faster = more effective collisions w/ more energy rxn rate increase Surface Area Concentration Nature of reactants/products Catalyst

Decrease in temp = particles move slower = less collisions w/ less energy rxn rate decreases Less surface area = less area exposed for a collision lower rxn rate Factor Increase Temperature Increase temp = particles move faster = more effective collisions w/ more energy rxn rate increase Surface Area More surface area = more area exposed for collisions higher rxn rate Concentration Nature of reactants/products Catalyst

Decrease in temp = particles move slower = less collisions w/ less energy rxn rate decreases Less surface area = less area exposed for a collision lower rxn rate Less particles means less odds that a collision will occur lower rxn rate Factor Increase Temperature Increase temp = particles move faster = more effective collisions w/ more energy rxn rate increase Surface Area More surface area = more area exposed for collisions higher rxn rate Concentration More particles means w/ more random motion = more collisions increase rxn rate Nature of reactants/products Catalyst

Decrease in temp = particles move slower = less collisions w/ less energy rxn rate decreases Less surface area = less area exposed for a collision lower rxn rate Less particles means less odds that a collision will occur lower rxn rate More complex = less likely to be effective Different polarities will not even get near to react decrease rxn rate Factor Increase Temperature Increase temp = particles move faster = more effective collisions w/ more energy rxn rate increase Surface Area More surface area = more area exposed for collisions higher rxn rate Concentration More particles means w/ more random motion = more collisions increase rxn rate Nature of reactants/products Simple = more likely to react = more effective collision Same polarities = will react Increase rxn rate Catalyst

Decrease in temp = particles move slower = less collisions w/ less energy rxn rate decreases Less surface area = less area exposed for a collision lower rxn rate Less particles means less odds that a collision will occur lower rxn rate More complex = less likely to be effective Different polarities will not even get near to react decrease rxn rate Don’t have one = increases the amount of energy required to start rxn decreases rxn rate Factor Increase Temperature Increase temp = particles move faster = more effective collisions w/ more energy rxn rate increase Surface Area More surface area = more area exposed for collisions higher rxn rate Concentration More particles means w/ more random motion = more collisions increase rxn rate Nature of reactants/products Simple = more likely to react = more effective collision Same polarities = will react Increase rxn rate Catalyst Have one = lowers energy required to start a rxn rate increases

Rate of Reactions: Explosions Hunting the Elements (minute 47: 55 – 58)

Basic Equilibrium Reflect: After a while, what happened to the amount of water in each container? Therefore, what can we say about the AMOUNT of water transfer from each container?

Modeling a Reversible Reaction

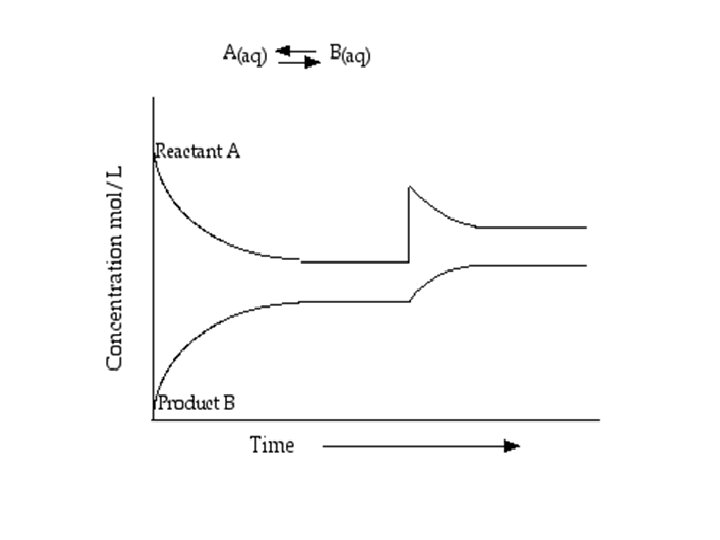
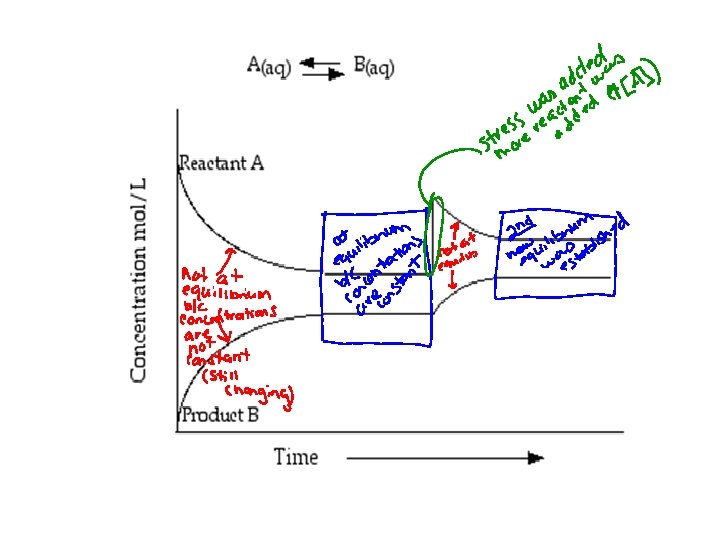
Equilibrium Dynamic Equilibrium: • When reactants and products turn into each other at EQUAL RATES (amount of time) • There is NO NET change (the dynamic part means BOTH processes are still occurring) The concentration of products and reactants are constant The rates are equal (amt. of R P and R P over the same amt. of time)
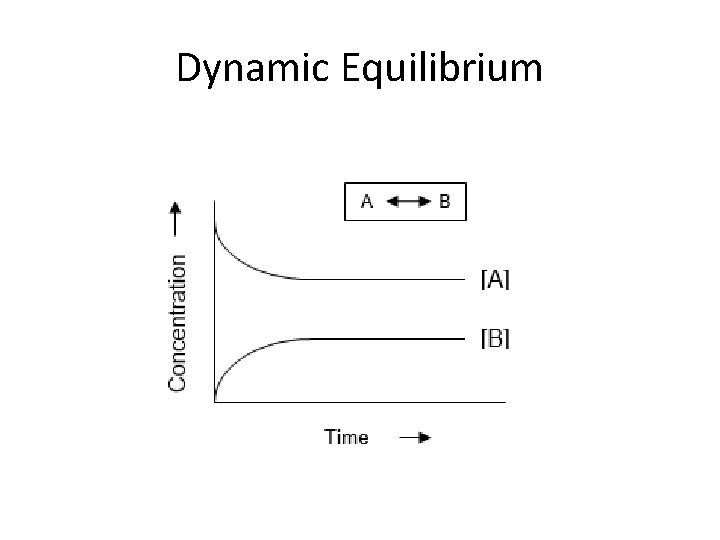
Dynamic Equilibrium
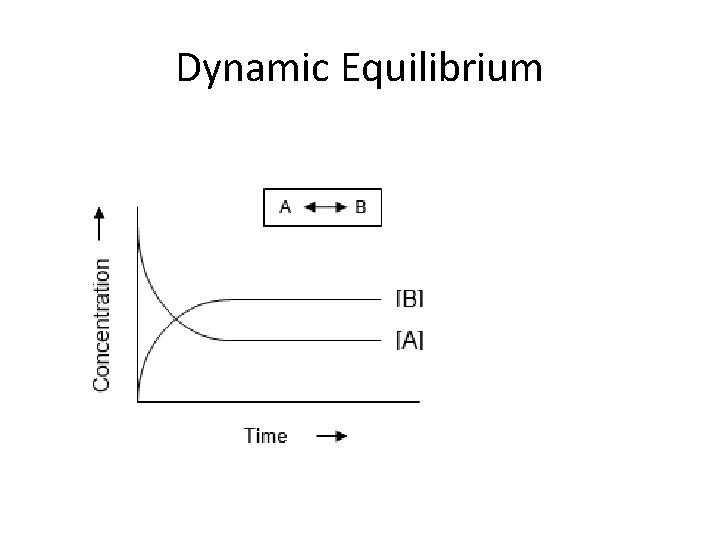
Dynamic Equilibrium

Dynamic Equilibrium

Equilibrium Chemical Equilibrium: Reactions that can occur in two directions (forward and reverse)
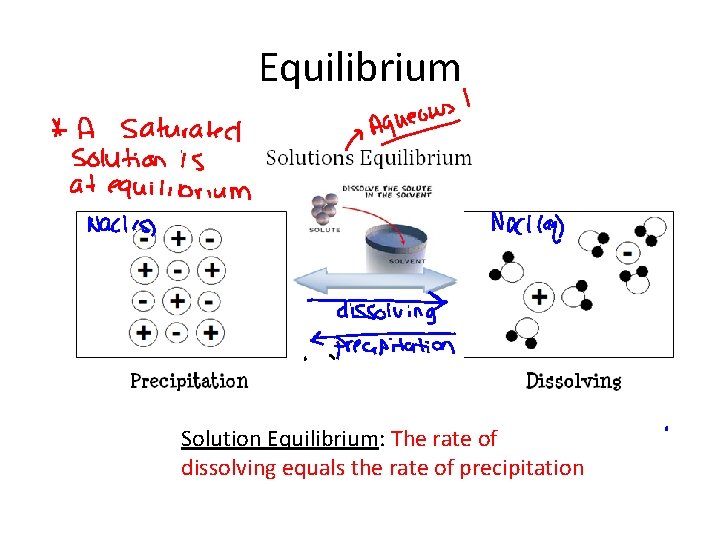
Equilibrium Solution Equilibrium: The rate of dissolving equals the rate of precipitation

Equilibrium Phase Equilibrium: When two phases exist simultaneously.

Basic Equilibrium Reversible Dynamic Equilibrium: https: //www. youtube. com/watch? v=wl. D_Im. YQ Ag. Q

Equilibrium *At equilibrium the rate of the forward reaction equals the rate of the reverse reaction. * The measurable quantities of reactants and products remain constant at equilibrium.
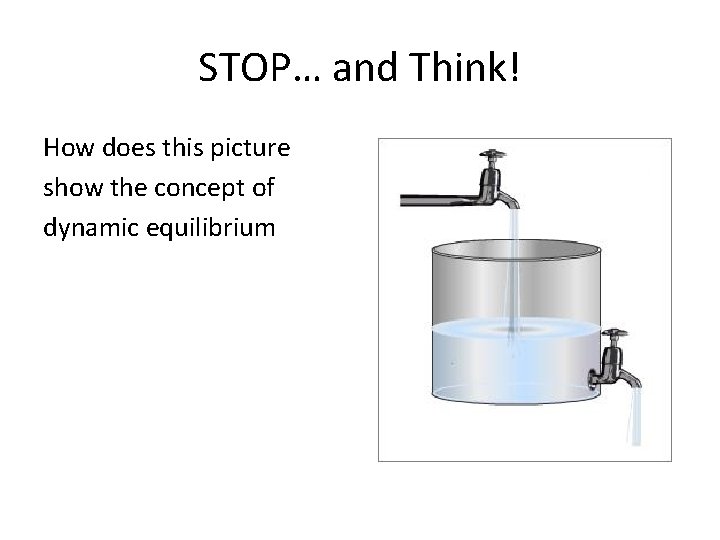
STOP… and Think! How does this picture show the concept of dynamic equilibrium

Rate of sale of cookies = Rate of replacing cookies

Le. Chatelier’s Principle: https: //www. youtube. com/watch? v=wl. D_Im. YQ Ag. Q

Le Chatelier’s Principle
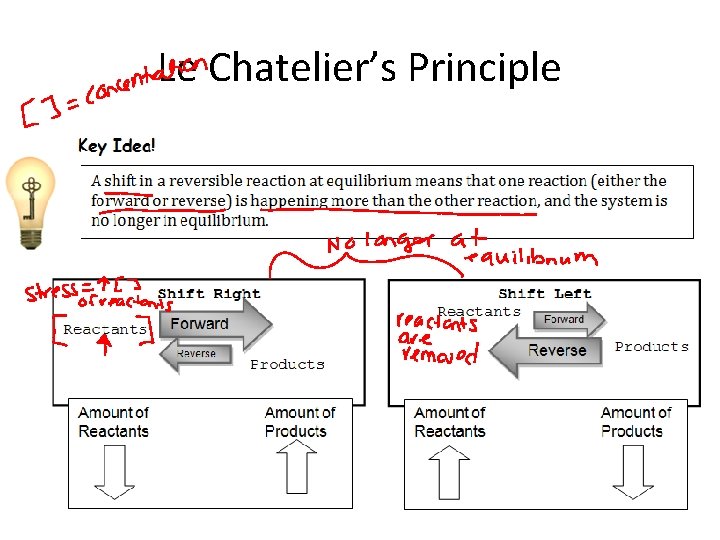
Le Chatelier’s Principle

Le Chaterlier’s Principle

Reaction Stresses Shifts in Equilibrium: When a reversible reaction is disrupted and will then proceed more towards the reactants or products.

Shifts in Equilibrium • Changes in Concentration: – Increase in concentration = more particles to collide so reaction will move in the direction that gets rid of the excess – Decrease in concentration = reaction will move in the direction that makes more of it

Reaction Stresses • Change in Temperature: Exothermic reaction: Heat is a product. Endothermic reaction: Heat is a reactant. A + B AB + Heat - Forward reaction creates/releases heat - Reverse reaction uses/takes in heat
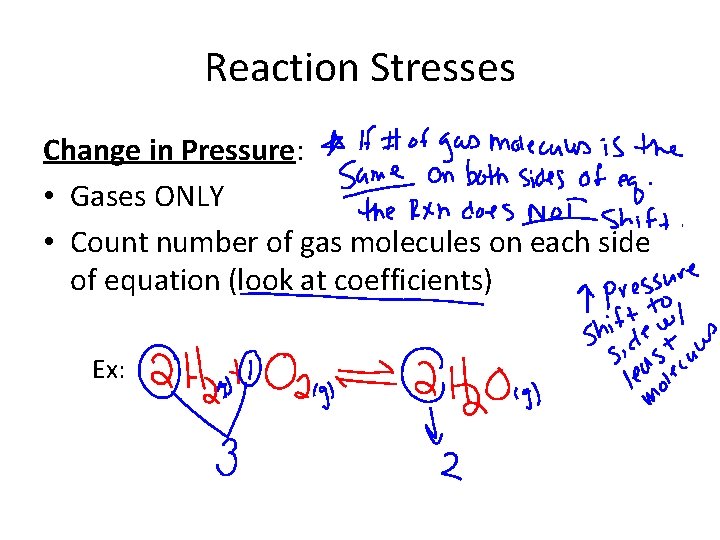
Reaction Stresses Change in Pressure: • Gases ONLY • Count number of gas molecules on each side of equation (look at coefficients) Ex:

Reaction Stresses

Le Chatelier’s Principle Ø When you ADD a substance: Ø When you REMOVE a substance:

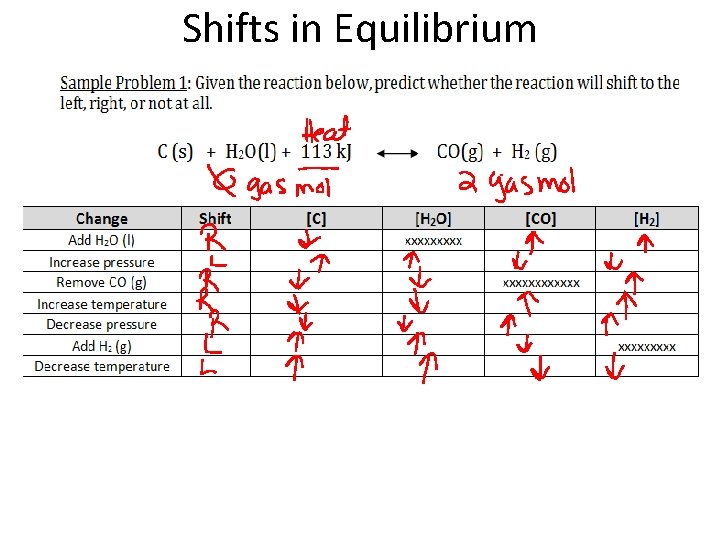
Shifts in Equilibrium Sample Problem 1: Given the reaction below, predict whether the reaction will shift to the left, right, or not at all. C (s) + H 2 O(l) + 113 k. J CO(g) + H 2 (g) • Increase in [H 2] = reaction shifts ____ • Decrease in [H 2] = reaction shifts ____
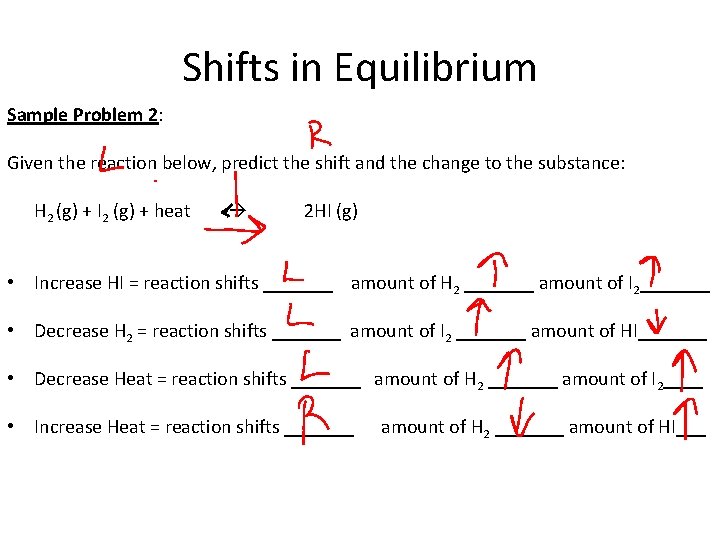
Shifts in Equilibrium Sample Problem 2: Given the reaction below, predict the shift and the change to the substance: H 2 (g) + I 2 (g) + heat 2 HI (g) • Increase HI = reaction shifts _______ amount of H 2 _______ amount of I 2_______ • Decrease H 2 = reaction shifts _______ amount of I 2 _______ amount of HI_______ • Decrease Heat = reaction shifts _______ amount of H 2 _______ amount of I 2____ • Increase Heat = reaction shifts _______ amount of H 2 _______ amount of HI___
Shifts in Equilibrium
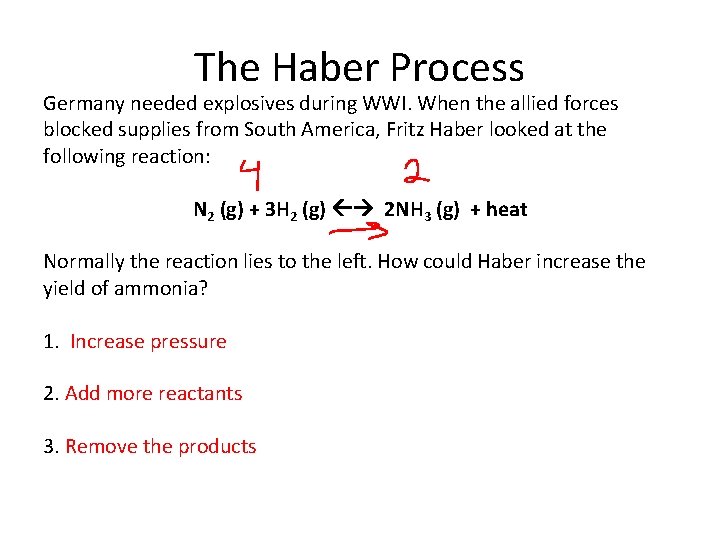
The Haber Process Germany needed explosives during WWI. When the allied forces blocked supplies from South America, Fritz Haber looked at the following reaction: N 2 (g) + 3 H 2 (g) 2 NH 3 (g) + heat Normally the reaction lies to the left. How could Haber increase the yield of ammonia? 1. Increase pressure 2. Add more reactants 3. Remove the products

The Haber Process Haber received the Nobel prize in 1918 (although he was expelled from Germany in 1933 because he was Jewish). We get nitrogen for fertilizers and cleaning products from this process.

The Haber Process http: //www. youtube. com/watch? v=td. EE 5 uv. Fh. OM

Le Châtelier’s Principle If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position. • Changes in Concentration N 2 (g) + 3 H 2 (g) 2 NH 3 (g)
- Slides: 45