Unit 8 Ionic Bonds Chapter 8 Chemistry 1
Unit 8: Ionic Bonds Chapter 8 Chemistry 1 L Cypress Creek High School

Part 1: Introducing Ionic Bonds
Chemical Bonds • A bond is a force that holds atoms together; creates a compound with new properties which differ from the individual elements – Chemical bonds form because of attractions between oppositely charged atoms, called ions • Octet Rule – Atoms bond because they are trying to achieve stable electron configurations like the noble gases (8 valence electrons)
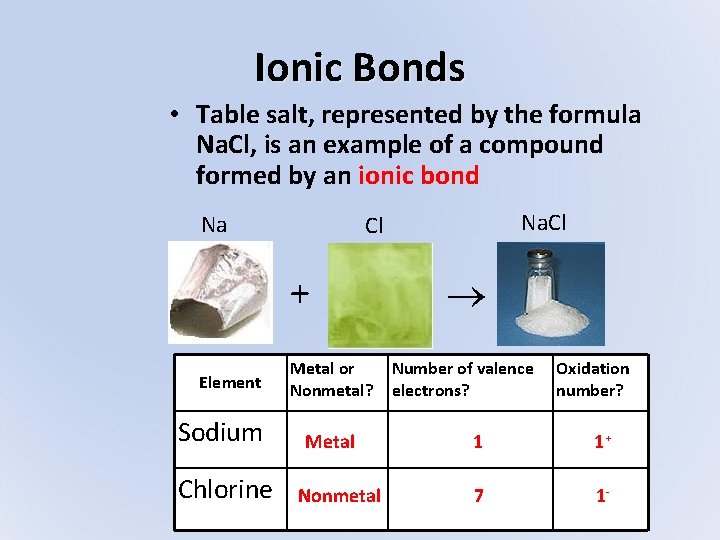
Ionic Bonds • Table salt, represented by the formula Na. Cl, is an example of a compound formed by an ionic bond Na + Element Sodium Chlorine Na. Cl Cl Metal or Nonmetal? Metal Nonmetal Number of valence electrons? Oxidation number? 1 1+ 7 1 -

Common Ionic Compounds Sodium chloride (Na. Cl) is table salt used in food Sodium bicarbonate (Na. HCO 3) is baking soda used in food, cleaning products, and antacids Sodium hydroxide (Na. OH) is lye used in soaps and drain cleaner Potassium nitrate (KNO 3) is saltpeter used in fertilizer, gunpowder, and food preservatives
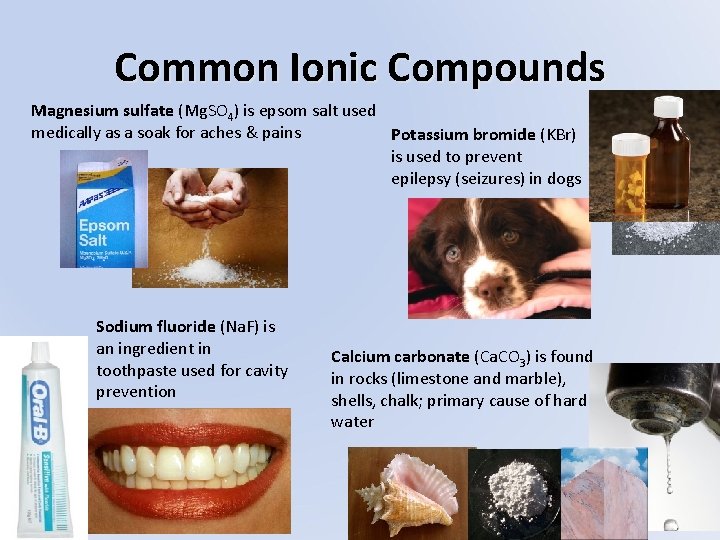
Common Ionic Compounds Magnesium sulfate (Mg. SO 4) is epsom salt used medically as a soak for aches & pains Potassium bromide (KBr) is used to prevent epilepsy (seizures) in dogs Sodium fluoride (Na. F) is an ingredient in toothpaste used for cavity prevention Calcium carbonate (Ca. CO 3) is found in rocks (limestone and marble), shells, chalk; primary cause of hard water
Ionic Compound Properties • • • Also known as salts A single entity is called a formula unit Exist as solids at room temperature Easily dissolve in water Exhibit a crystal structure
Ionic Compound Properties • Possess high melting / boiling points • Considered an electrolyte – a compound that conducts electricity in solution (dissolved in water) • Have high electronegativity differences between ions
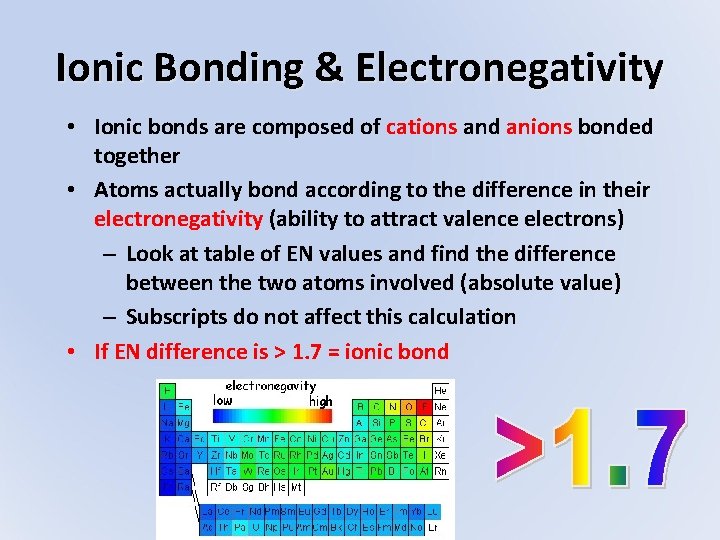
Ionic Bonding & Electronegativity • Ionic bonds are composed of cations and anions bonded together • Atoms actually bond according to the difference in their electronegativity (ability to attract valence electrons) – Look at table of EN values and find the difference between the two atoms involved (absolute value) – Subscripts do not affect this calculation • If EN difference is > 1. 7 = ionic bond
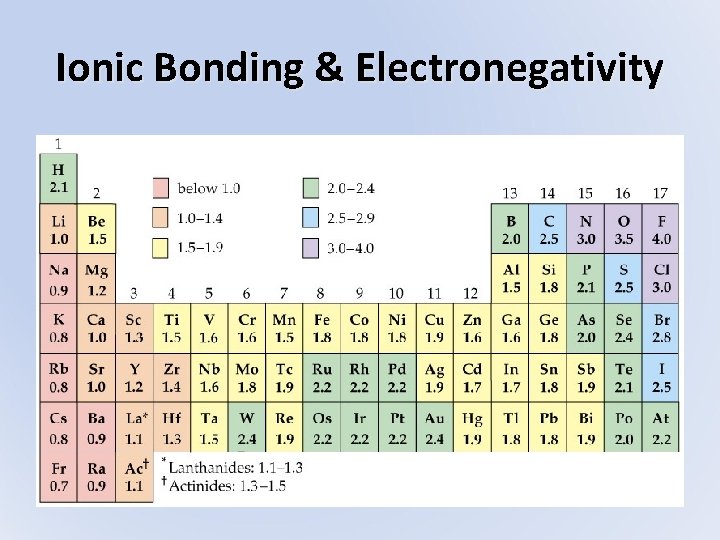
Ionic Bonding & Electronegativity
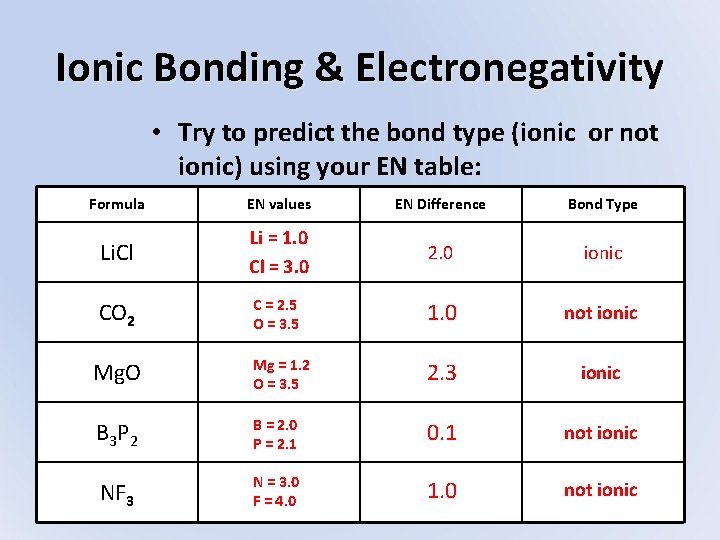
Ionic Bonding & Electronegativity • Try to predict the bond type (ionic or not ionic) using your EN table: Formula EN values EN Difference Bond Type Li. Cl Li = 1. 0 Cl = 3. 0 2. 0 ionic CO 2 C = 2. 5 O = 3. 5 1. 0 not ionic Mg. O Mg = 1. 2 O = 3. 5 2. 3 ionic B 3 P 2 B = 2. 0 P = 2. 1 0. 1 not ionic NF 3 N = 3. 0 F = 4. 0 1. 0 not ionic
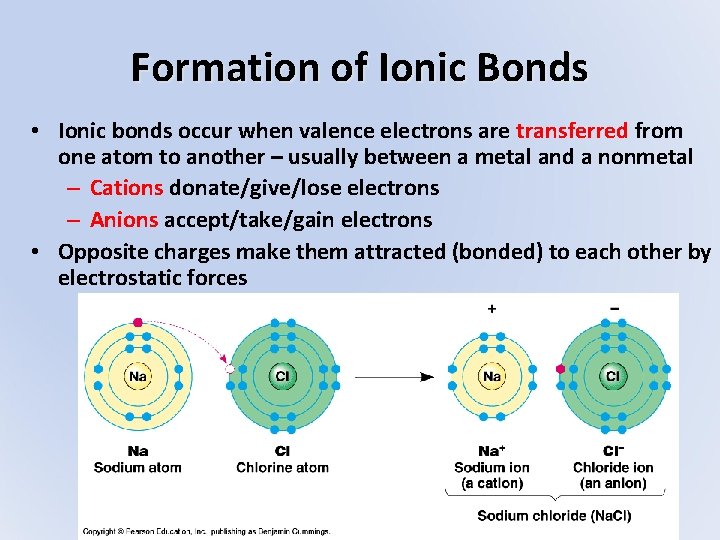
Formation of Ionic Bonds • Ionic bonds occur when valence electrons are transferred from one atom to another – usually between a metal and a nonmetal – Cations donate/give/lose electrons – Anions accept/take/gain electrons • Opposite charges make them attracted (bonded) to each other by electrostatic forces
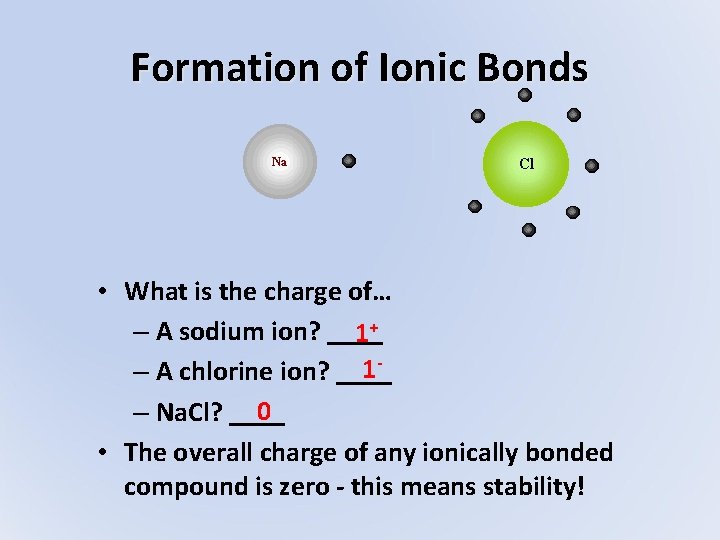
Formation of Ionic Bonds • What is the charge of… – A sodium ion? ____ 1+ 1– A chlorine ion? ____ 0 – Na. Cl? ____ • The overall charge of any ionically bonded compound is zero - this means stability!
- Slides: 14