Unit 8 Electrostatics Electrostatics interactions between electric charges

Unit 8 Electrostatics
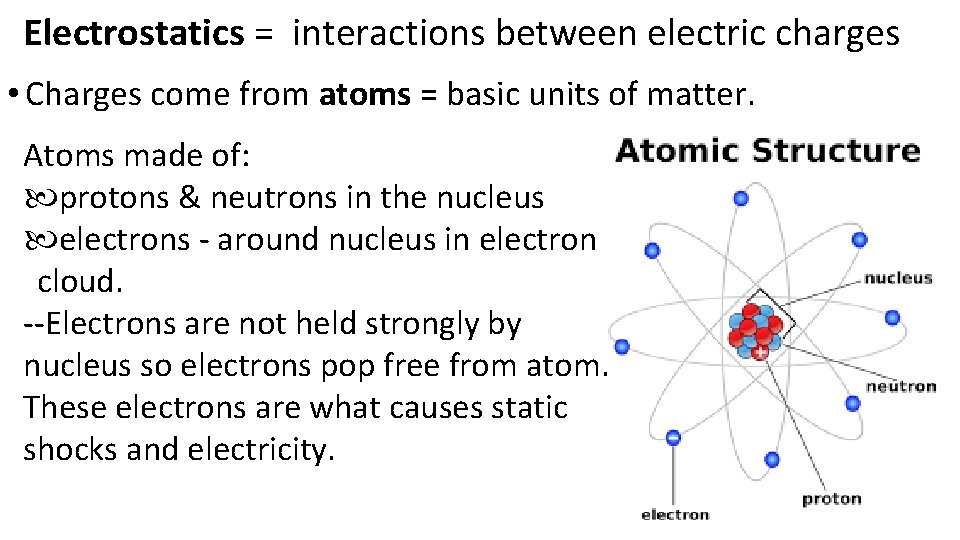
Electrostatics = interactions between electric charges • Charges come from atoms = basic units of matter. Atoms made of: protons & neutrons in the nucleus electrons - around nucleus in electron cloud. --Electrons are not held strongly by nucleus so electrons pop free from atom. These electrons are what causes static shocks and electricity.
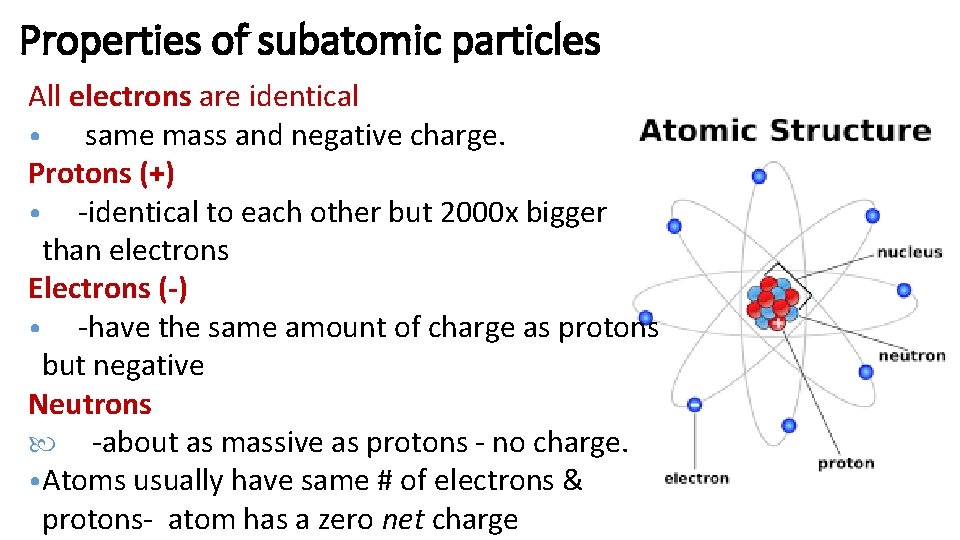
Properties of subatomic particles All electrons are identical • same mass and negative charge. Protons (+) • -identical to each other but 2000 x bigger than electrons Electrons (-) • -have the same amount of charge as protons but negative Neutrons -about as massive as protons - no charge. • Atoms usually have same # of electrons & protons- atom has a zero net charge
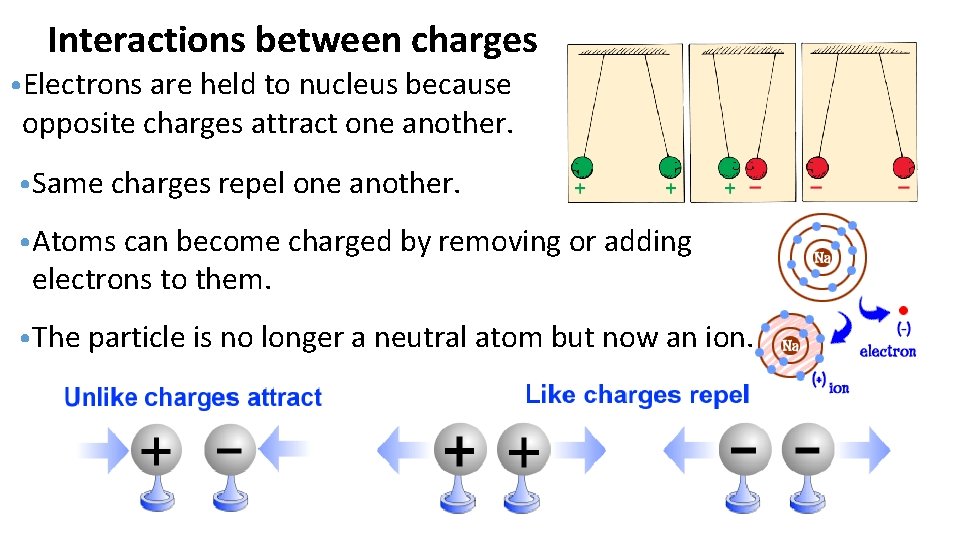
Interactions between charges • Electrons are held to nucleus because opposite charges attract one another. • Same charges repel one another. • Atoms can become charged by removing or adding electrons to them. • The particle is no longer a neutral atom but now an ion.
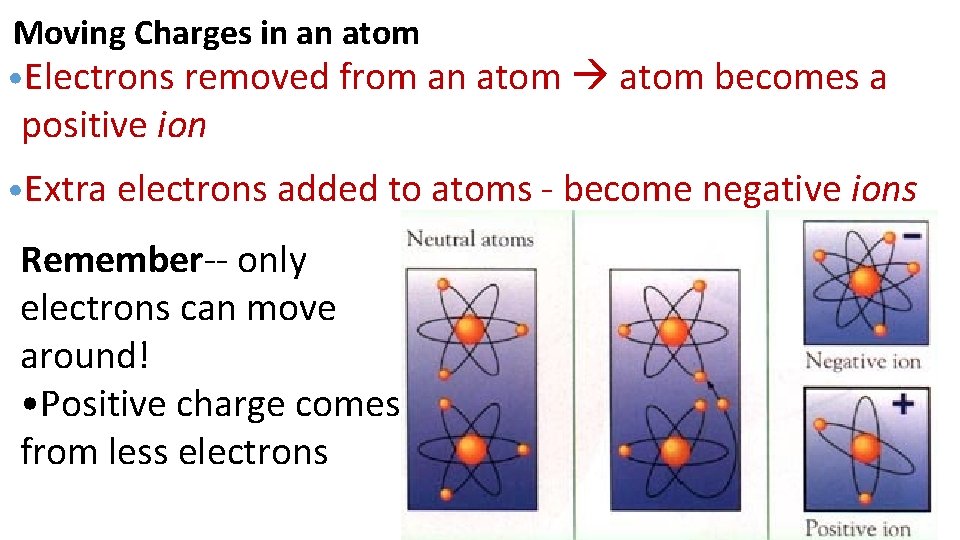
Moving Charges in an atom • Electrons removed from an atom becomes a positive ion • Extra electrons added to atoms - become negative ions Remember-- only electrons can move around! • Positive charge comes from less electrons

Conservation of charge • Charge is about electrons moving from one place to another. • All electrons have to be accounted for. called the conservation of charge. Net electrical charge is neither created nor destroyed but is transferable from one material to another. If you scuff electrons onto your shoe as you walk across the carpet, do you become more positively charged or negatively charged? simulation
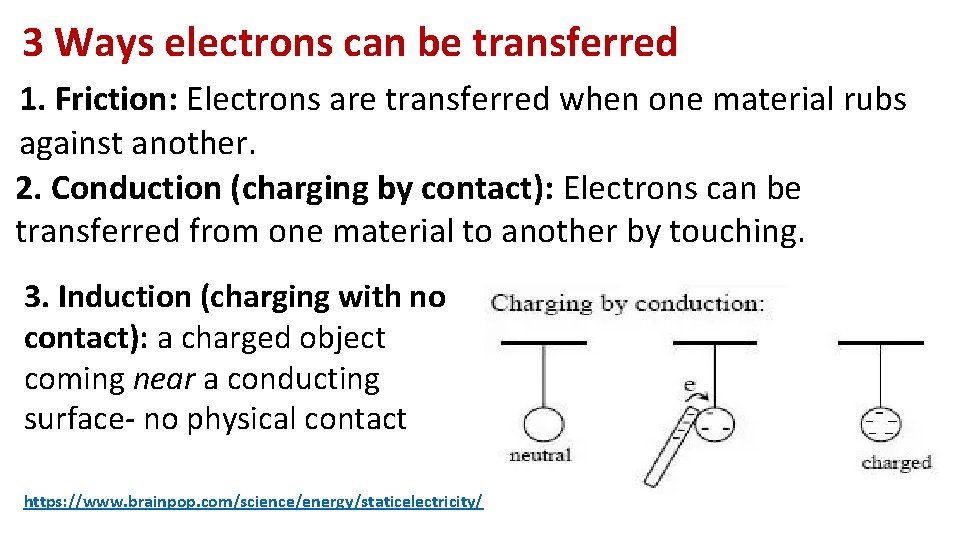
3 Ways electrons can be transferred 1. Friction: Electrons are transferred when one material rubs against another. 2. Conduction (charging by contact): Electrons can be transferred from one material to another by touching. 3. Induction (charging with no contact): a charged object coming near a conducting surface- no physical contact https: //www. brainpop. com/science/energy/staticelectricity/

How can we move charges from one object to another? Conductors: • Materials –electric charges flow easily • Electrons of atoms in metal not attached to nuclei - free to roam in material. • Metals are good conductors for electric charges - their electrons are “loose. ” Insulators: • Materials are poor conductors of electricity • Electrons in materials = rubber and glass—are tightly bound and stay with atoms. • Electrons are not free to wander to other atoms in material.
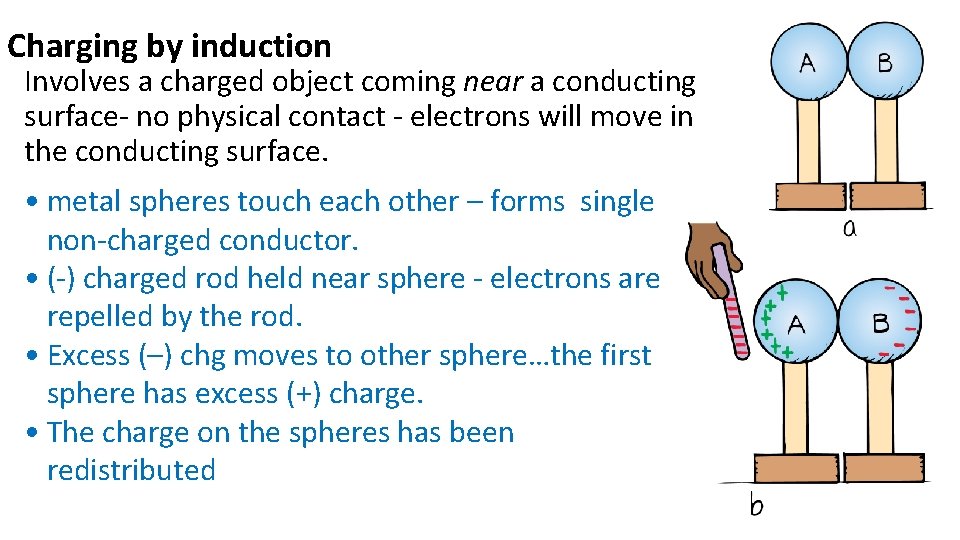
Charging by induction Involves a charged object coming near a conducting surface- no physical contact - electrons will move in the conducting surface. • metal spheres touch each other – forms single non-charged conductor. • (-) charged rod held near sphere - electrons are repelled by the rod. • Excess (–) chg moves to other sphere…the first sphere has excess (+) charge. • The charge on the spheres has been redistributed
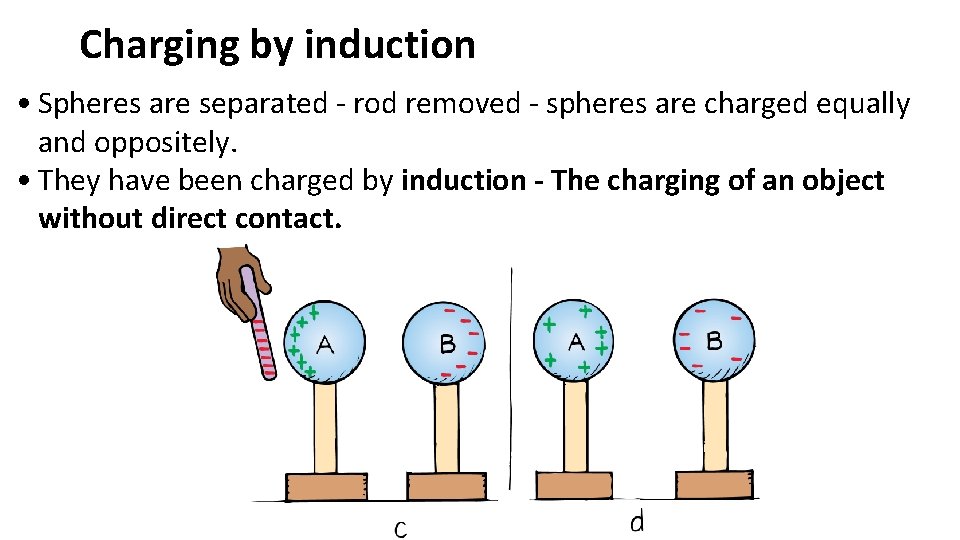
Charging by induction • Spheres are separated - rod removed - spheres are charged equally and oppositely. • They have been charged by induction - The charging of an object without direct contact.
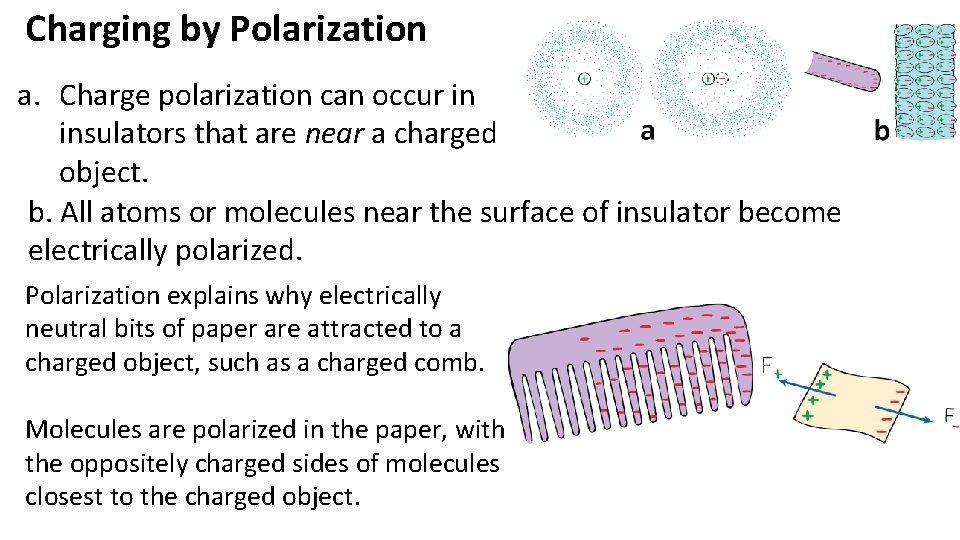
Charging by Polarization a. Charge polarization can occur in insulators that are near a charged object. b. All atoms or molecules near the surface of insulator become electrically polarized. Polarization explains why electrically neutral bits of paper are attracted to a charged object, such as a charged comb. Molecules are polarized in the paper, with the oppositely charged sides of molecules closest to the charged object.
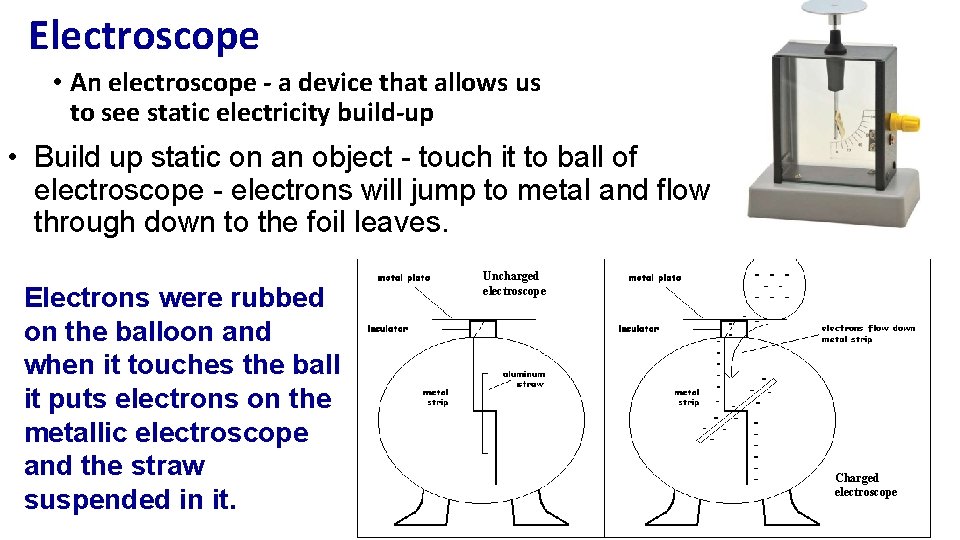
Electroscope • An electroscope - a device that allows us to see static electricity build-up • Build up static on an object - touch it to ball of electroscope - electrons will jump to metal and flow through down to the foil leaves. Electrons were rubbed on the balloon and when it touches the ball it puts electrons on the metallic electroscope and the straw suspended in it. Uncharged electroscope Charged electroscope

Day 1 – Stop here! Complete Study Guide Questions A – G You may need to refer to the textbook pages for more explanation: Click Here: Electrostatic Text Ch 32
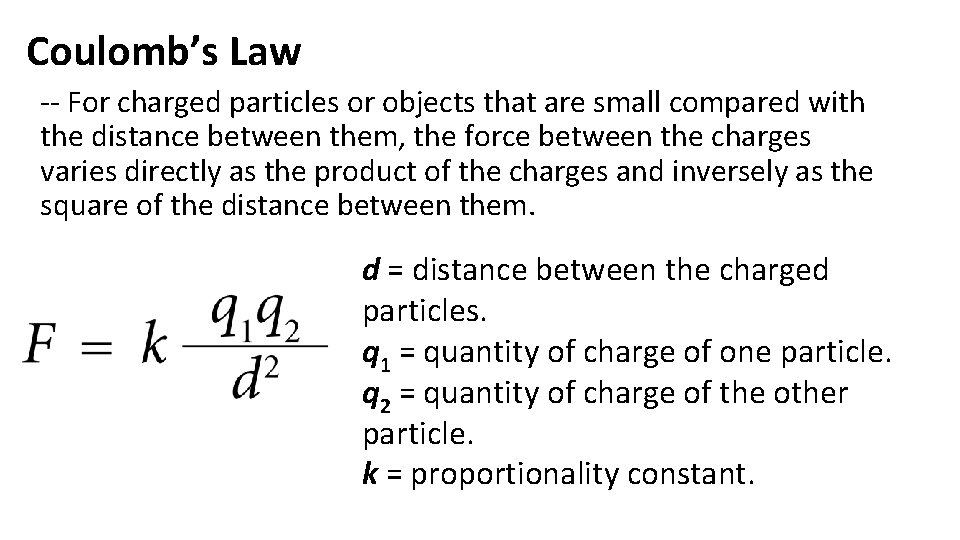
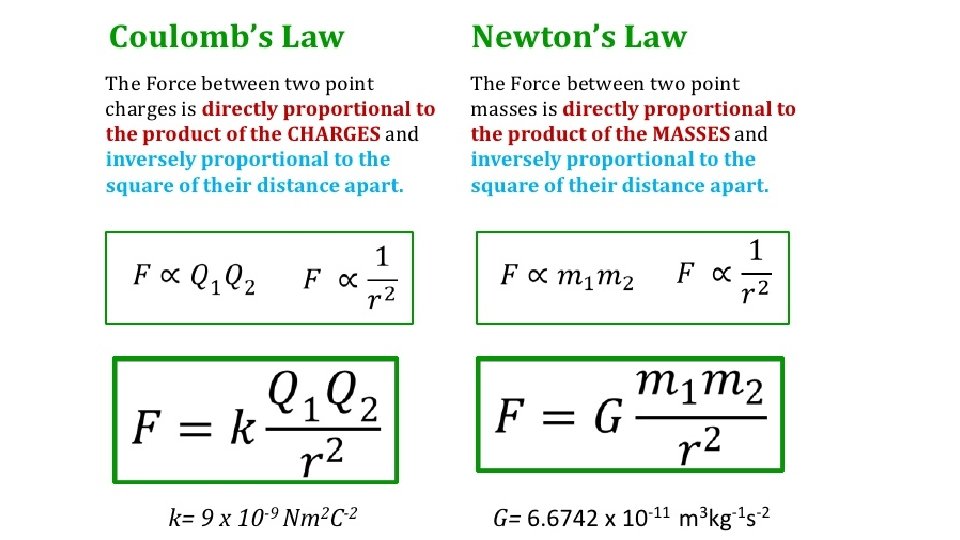
Coulomb’s Law -- For charged particles or objects that are small compared with the distance between them, the force between the charges varies directly as the product of the charges and inversely as the square of the distance between them. d = distance between the charged particles. q 1 = quantity of charge of one particle. q 2 = quantity of charge of the other particle. k = proportionality constant.
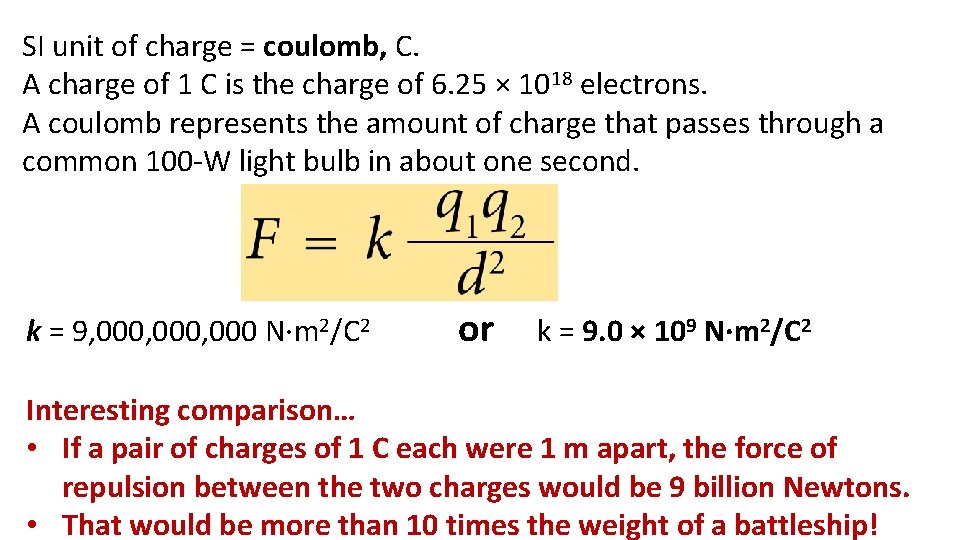
SI unit of charge = coulomb, C. A charge of 1 C is the charge of 6. 25 × 1018 electrons. A coulomb represents the amount of charge that passes through a common 100 -W light bulb in about one second. k = 9, 000, 000 N·m 2/C 2 or k = 9. 0 × 109 N·m 2/C 2 Interesting comparison… • If a pair of charges of 1 C each were 1 m apart, the force of repulsion between the two charges would be 9 billion Newtons. • That would be more than 10 times the weight of a battleship!
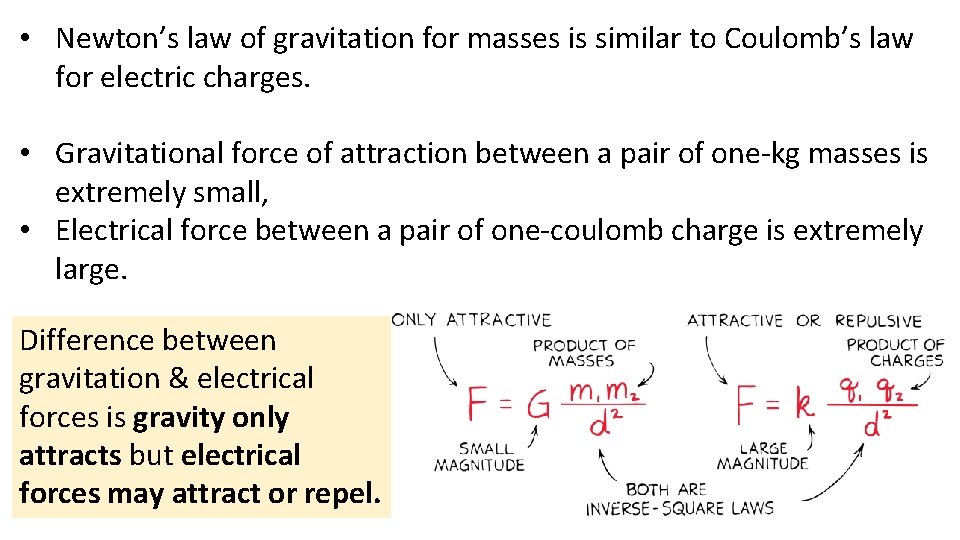
• Newton’s law of gravitation for masses is similar to Coulomb’s law for electric charges. • Gravitational force of attraction between a pair of one-kg masses is extremely small, • Electrical force between a pair of one-coulomb charge is extremely large. Difference between gravitation & electrical forces is gravity only attracts but electrical forces may attract or repel.
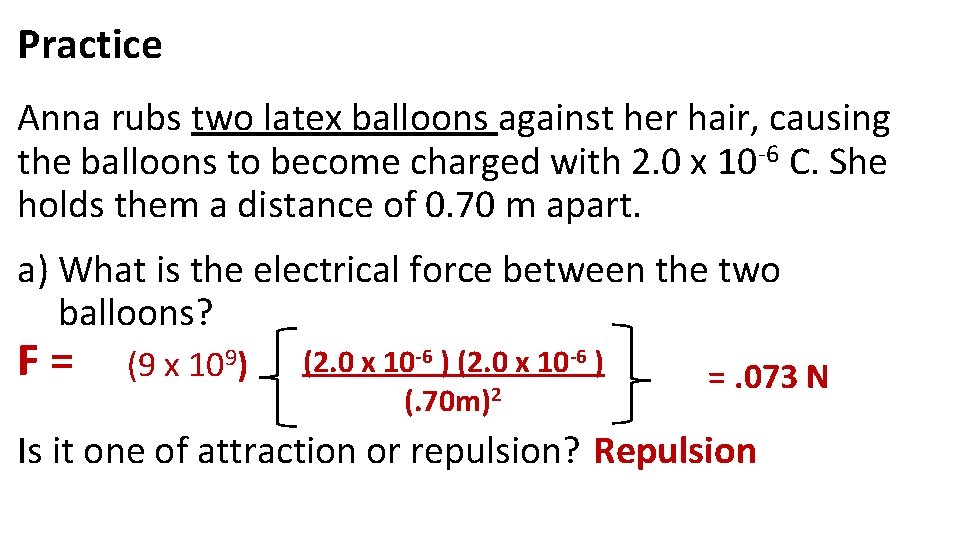
Practice Anna rubs two latex balloons against her hair, causing the balloons to become charged with 2. 0 x 10 -6 C. She holds them a distance of 0. 70 m apart. a) What is the electrical force between the two balloons? F= (9 x 109) (2. 0 x 10 -6 ) (. 70 m)2 =. 073 N Is it one of attraction or repulsion? Repulsion

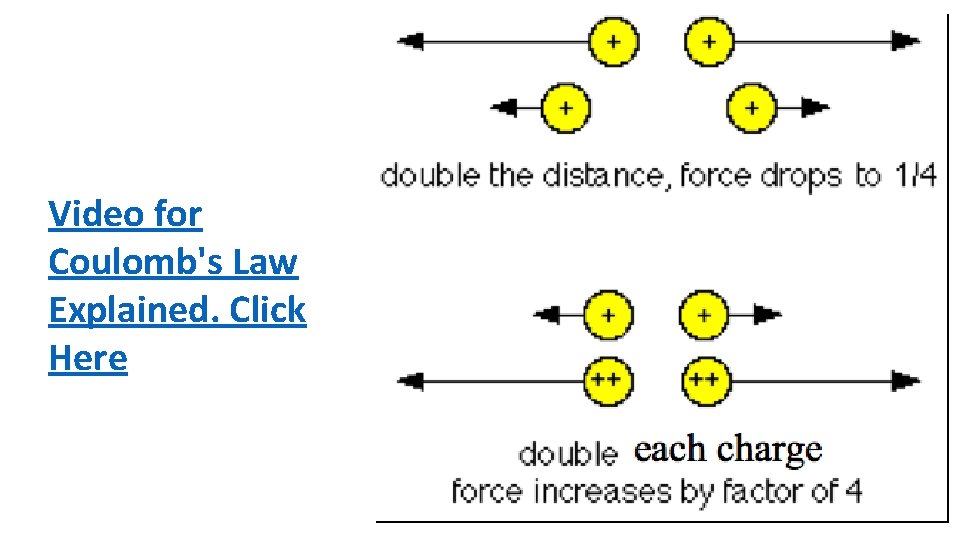
Coulomb’s law Think! If an electron at a certain distance from a charged particle is attracted with a certain force, how will the force compare at twice this distance? Answer: ----inverse-square law, at twice the distance the force will be one fourth as much.
Coulomb’s law Think! What is the main significance of the fact that G in Newton’s law of gravitation is a small number and k in Coulomb’s law is a large number when both are expressed in SI units? Answer: The small value of G indicates that gravity is a weak force; the large value of k indicates that the electrical force is enormous in comparison.

Coulomb’s Law-Explained by Mr. Teters Click Here for Coulomb's Law explained
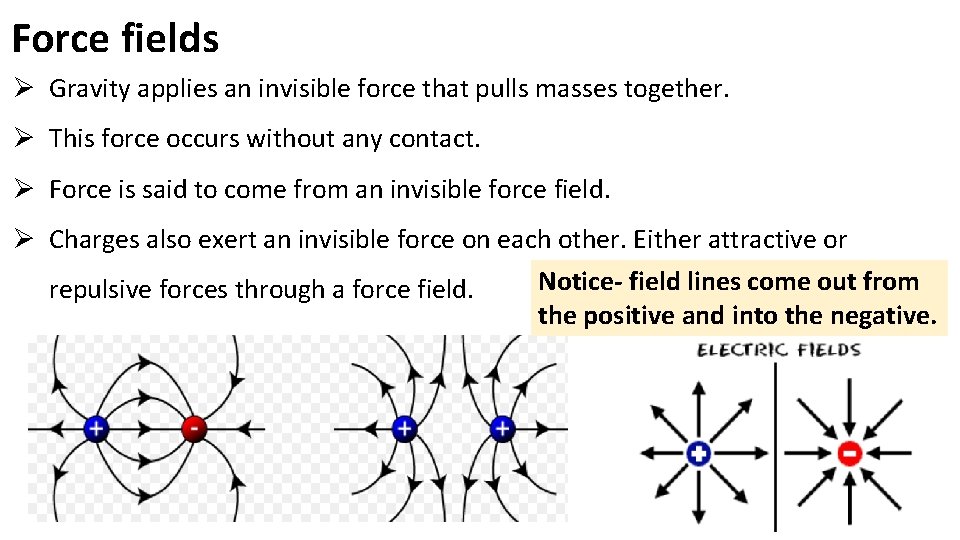
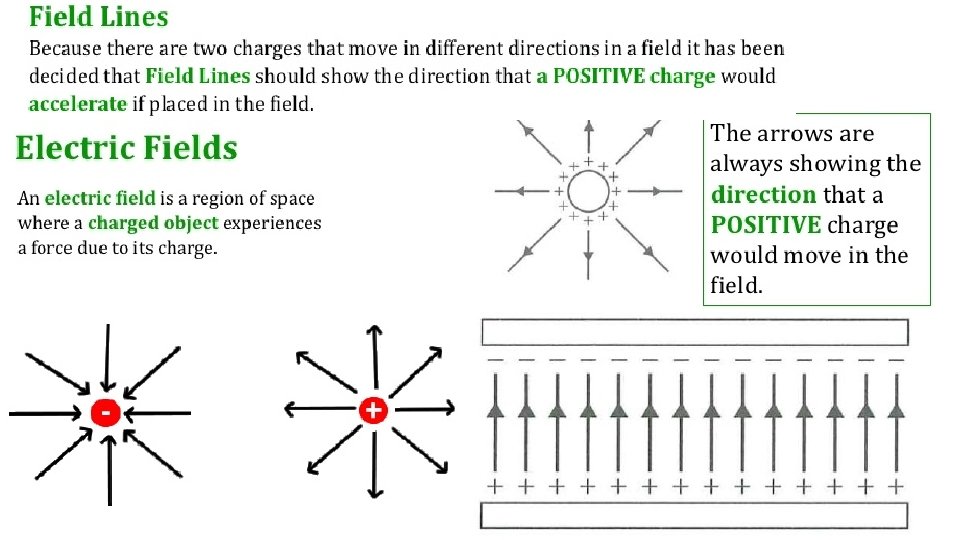
Force fields Ø Gravity applies an invisible force that pulls masses together. Ø This force occurs without any contact. Ø Force is said to come from an invisible force field. Ø Charges also exert an invisible force on each other. Either attractive or Notice- field lines come out from repulsive forces through a force field. the positive and into the negative.
Video for Coulomb's Law Explained. Click Here
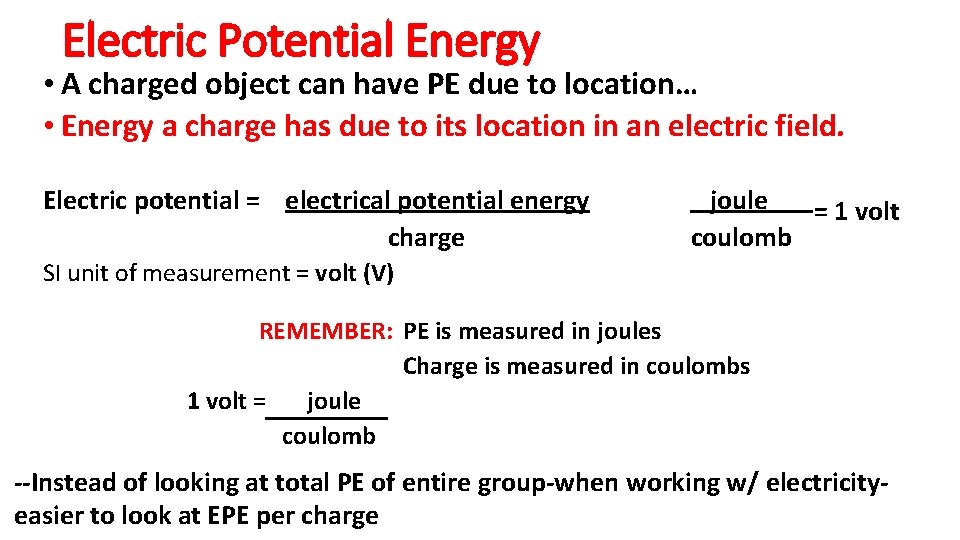
Electric Potential Energy • A charged object can have PE due to location… • Energy a charge has due to its location in an electric field. Electric potential = electrical potential energy charge joule = 1 volt coulomb SI unit of measurement = volt (V) REMEMBER: PE is measured in joules Charge is measured in coulombs 1 volt = joule coulomb --Instead of looking at total PE of entire group-when working w/ electricityeasier to look at EPE per charge

Electric Potential Energy - Explained by Mr. Teters Click Here for Electric Potential Energy explained
https: //www. express. co. uk/life-style/life/831581/viral-videoslightning-strikes

Complete the rest of the study guide. You may need to refer to the textbook pages for more explanation: • Click Here: Ch 33 Electric Potential Text
- Slides: 29