Unit 8 Covalent Bonding To bond or not

Unit 8 – Covalent Bonding

To bond or not to bond? • What is a chemical bond? • Forces of attraction that hold groups of atoms together • Why do elements form bonds? • Octet Rule • Atoms gain, lose or share to have eight electrons on the outer shell

Types of Bonds • Ionic Bond • Covalent Bond

Ionic Bonds • Between a METAL and a NONMETAL • Between a CATION and an ANION • Most are CRYSTALLINE solids • High melting points • Good conductors • They are called “salts”

Covalent Bonds • Between a NONMETAL and a NONMETAL • They are called molecules • Low melting points • Involve the sharing of electrons • Non-polar- equal sharing of electrons • Polar- unequal sharing of electrons
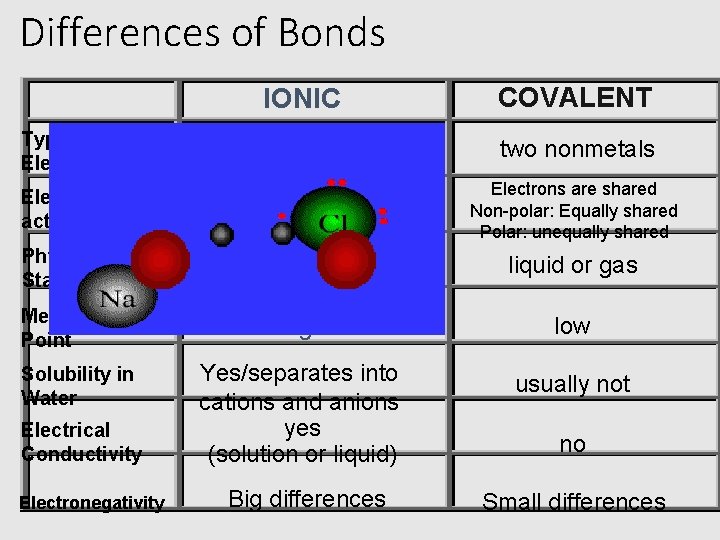
Differences of Bonds IONIC COVALENT Types of Elements metal and nonmetal two nonmetals Electron activity Electrons transfer Metals lose=cations Non-metals gain=anions Electrons are shared Non-polar: Equally shared Polar: unequally shared Physical State solid liquid or gas Melting Point high low Yes/separates into cations and anions yes (solution or liquid) usually not Solubility in Water Electrical Conductivity Electronegativity Big differences no Small differences

Naming Covalent Compounds • Only use a prefix with the first element when there is more than one. • ALWAYS use a prefix with the second element and ends in -ide • One- mono Six- hexa • Two- di Seven- hepta • Three- tri Eight- octa • Four- tetra Nine- nona • Five- penta Ten- deca

• 11 – undeca • 12 – dodeca • 13 – trideca • 14 – tetradeca • 15 – pentadeca • 16 – hexadeca • 17 – heptadeca • 18 – octadeca • 19 - nonadeca

Practice Naming Covalent Compounds • CO 2 • N 2 O 5 • NF 3

Writing Covalent Compounds • Write the symbol of the first element. • If it has a prefix, put the number after the element as subscript. • Write the symbol of the second element • Put the number represented by the prefix after it as a subscript. • DO NOT REDUCE! Leave it as it is.

Practice Writing Covalent Compounds • Iodine pentafluoride • Nitrogen tribromide • Diphosphorus pentoxide • Sulfur hexachloride

Activity • Your partner is the person across from you. • Set of cards with 20 items • Sort them into “Ionic” and “Covalent” categories • See if you can find all the matches

Diatomic Molecules • Molecule: another name for a compound which has covalent bonds • Diatomic molecule: covalent molecule with two of the same atoms • Seven you need to know: I 2, Br 2, Cl 2, F 2, O 2, N 2, H 2

Lewis Dot for Covalent Compounds • Lewis Dot Review: What is a Lewis dot diagram? • Draw the Lewis dot for the following: S N C Xe He

Bell Work 11/10/14 • Log on to socrative. com • Enter Room number 4 f 6 c 8 fbc • Wait for teacher to begin activity • Get out your notes AGENDA Review Lewis Dot Rules for Covalent Molecules Examples Activity - Practice Socrative Exit Ticket

Review • Triselenium tetranitride • Tetracarbon Octahydride • Lewis Dot for: P • What happens to electrons in covalent bonds?

Lewis Dot for Covalent Molecules • Draw the Lewis dot diagram so every element is SHARING 8 valence electrons. • EXCEPTION #1: H only needs 2 valence electrons • EXCEPTION #2: Be, B, and other Group 3 elements not need 8 valence electrons • EXCEPTION #3: Some elements when bonded with certain other elements will have more than 8 valence electrons. Called “expanded octet. ” • Covalent molecules can create: • • Single bonds: sharing 2 electrons Double bonds: sharing 4 valence electrons (2 pairs) Triple bonds: sharing 6 valence electrons (3 pairs) Cannot share more than 6 valence electrons!!!

Examples:

Steps for Lewis Dot Structures 1. Determine the total number of valence electrons for all of the atoms in the formula 2. Use the electrons to satisfy all elements with 8 electrons (except for H, Be, B, and other Group 3 elements). 3. Share 2 electrons at a time (single bond) 4. When you run out of electrons, consider if you need to share more! * 4 electrons = double bond * 6 electrons = triple bond 5. If all elements are satisfied and there are leftover electrons, they are placed on cental atom (expanded octet).

EXIT TICKET • Socrative Quiz is Now Activated. • 8 questions based on today’s lecture • Good Luck!!!

How To Determine Shapes of Molecules: • The shape of a molecule can determine its physical and chemical properties. • The shapes determine whether they can get close enough to react.

VSEPR Theory V alence S hell E lectron P air R epulsion • repulsion between pairs of electrons around an atom cause them to be as far apart as possible • used to predict the geometry of molecules • Must first draw the CORRECT Lewis Dot Structure

Molecular Shapes • diatomic molecules will always be linear • all other molecules can have different shapes based on the number of charged electron clouds around the central atom • charge electron clouds include: • bonding pairs • lone pairs

Hybridization • Hybrid: Two things combined and the result characteristics of both. • Hybrid cars? • During bonding, atomic orbitals undergo hybridization • For example, when carbon bonds with four other atoms, the four valence electrons in the 2 s 22 p 2 orbitals hybridize to form four identical sp 3 hybrid orbitals.







2 Electron Clouds Around Central Atom • no lone pairs: linear • CO 2 O = C =O

3 Electron Clouds Around the Central Atom • no lone pairs: trigonal planar • CH 2 O • 1 lone pair: bent or angular • SO 2 O=S - O

4 Electron Clouds • no lone pairs: CH 4 tetrahedral • 1 lone pair: NH 3 pyramidal • 2 lone pairs: H 2 O bent or angular

5 Electron Clouds • no lone pairs: trigonal bipyramidal • PCl 5 • 1 lone pair: seesaw • SF 4

5 Electron Clouds • 2 lone pairs: • Cl. F 3 T-shaped • 3 lone pair: Linear • I 3 -

6 Electron Clouds • 2 lone pairs: square planar • Xe. F 4 • no lone pairs: octahedral • SF 6 Cl • 1 lone pair: square pyramidal • Sb. Cl 52 - Cl Cl Sb Cl Cl

Three Types of Bonds • Ionic – Electrons are _____ and _______ • Nonpolar Covalent – Electrons are ___________. • Polar Covalent – Electrons are ____________.

Polar vs. Nonpolar • Polar • Nonpolar • Definition: unequal • Definition: equal sharing of electrons • In a polar • Nonpolar molecule there molecule has 0 will be at least free pairs from one free pair for the central atom


Nonpolar/Polar/Ionic Bonds Difference in Electronegativity Type of Bond 0 – 0. 6 – 1. 8 + Non-Polar Ionic

Polar or Nonpolar Molecule? • Step 1 – Draw the Lewis dot for your molecule • Step 2 – Determine the hybridization, electronic and molecular geometry of the molecule • Step 3 – Determine if there are unshared pairs of electrons. • Step 4 -Determine if there is an overall positive and negative end to the molecule * If so, the molecule is Polar * If not, the molecule is Nonpolar Note: It is possible to have polar bonds, but nonpolar molecule

Intra Versus Inter • Intra? ? . . Within • Inter? ? . . Between or Among

Intramolecular Forces • These are the forces we have learned so far. • These are the chemical bonds • There are 3 types…. what are they again? ___________________ • These are stronger than intermolecular forces

Intermolecular Forces • Attraction between ________. • There are Four types of intermolecular forces (IMF).

IMF #1: Ionic Attraction • Involves Ionic Bonds which have a _____ and an ______ • The _____ end of one molecule is attracted to the ______ end of its neighbor.


IMF #2: Dipole-Dipole Attraction • Attraction between _______ molecules based upon a difference in _______. • The molecule must be _____ and be _____ (i. e. have a ____ pair of electrons)


IMF #3: Hydrogen Bonding • A special kind of dipole-dipole attraction involving _______ • Hydrogen MUST be bonded directly to: _________________


IMF #4: London Dispersion Force • A weak force that holds together molecules that are ________. • Typically happens at ______ temperatures.



EXAMPLES: • H 2 0 • N 2 • SCl 2 • H 2 S • Ca. Cl 2
- Slides: 54