Unit 8 Chemical Reactions Textbook Chapter 8 Day

Unit 8 Chemical Reactions Textbook Chapter 8
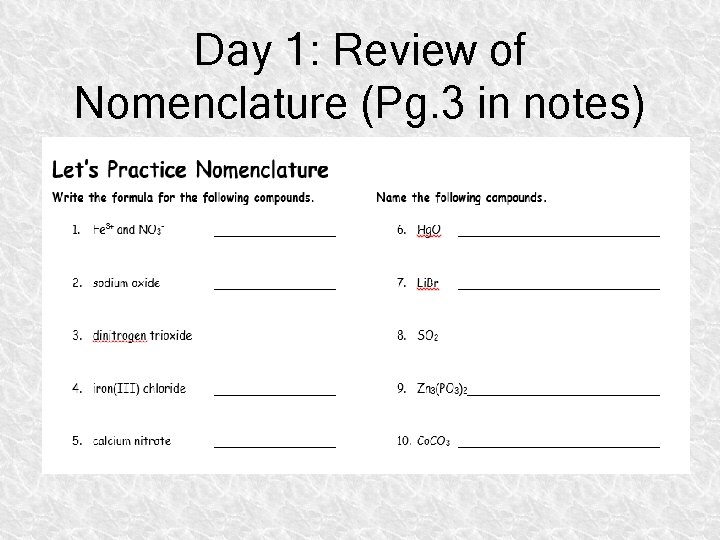
Day 1: Review of Nomenclature (Pg. 3 in notes)

Law of Conservation of Mass Experiment Matter can neither be created nor destroyed. Therefore, the amount of mass I start with will always equal the amount of mass I get back in any chemical equation!
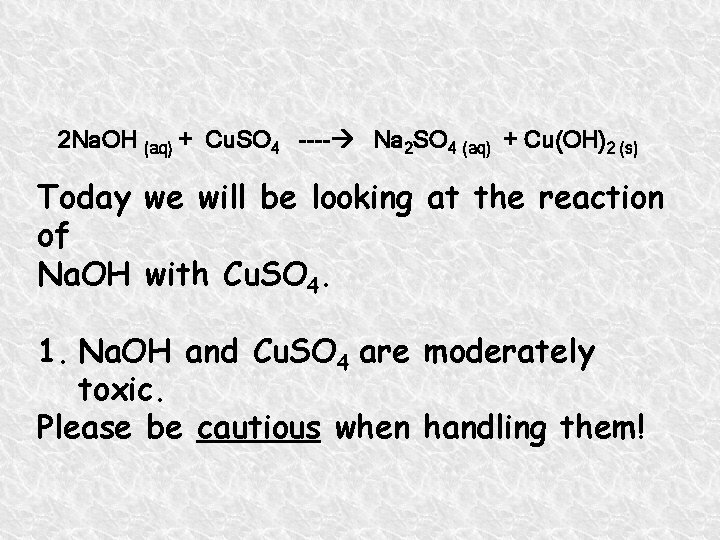
2 Na. OH (aq) + Cu. SO 4 ---- Na 2 SO 4 (aq) + Cu(OH)2 (s) Today we will be looking at the reaction of Na. OH with Cu. SO 4. 1. Na. OH and Cu. SO 4 are moderately toxic. Please be cautious when handling them!
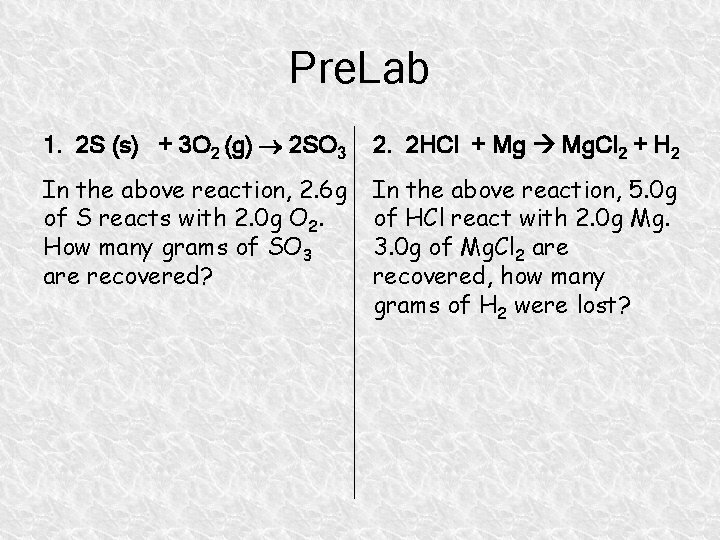
Pre. Lab 1. 2 S (s) + 3 O 2 (g) 2 SO 3 2. 2 HCl + Mg Mg. Cl 2 + H 2 In the above reaction, 2. 6 g of S reacts with 2. 0 g O 2. How many grams of SO 3 are recovered? In the above reaction, 5. 0 g of HCl react with 2. 0 g Mg. 3. 0 g of Mg. Cl 2 are recovered, how many grams of H 2 were lost?
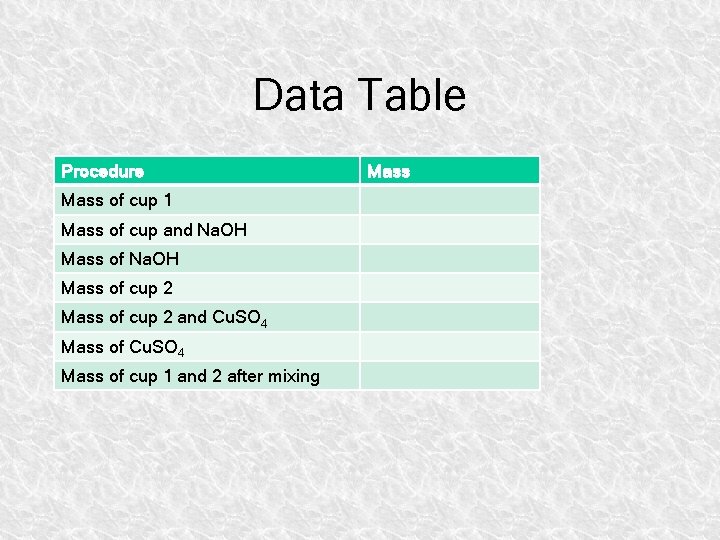
Data Table Procedure Mass of cup 1 Mass of cup and Na. OH Mass of cup 2 and Cu. SO 4 Mass of cup 1 and 2 after mixing Mass

Homework page 5

Day 2: Nomenclature Review • • • aluminum oxide ______ nitrogen trioxide ______ copper(II) nitrate ______ sodium carbonate____ lead(IV) oxide ____
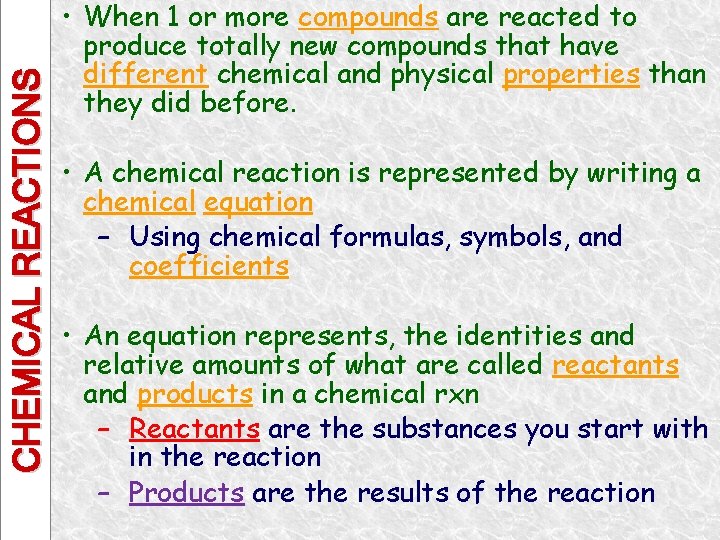
CHEMICAL REACTIONS • When 1 or more compounds are reacted to produce totally new compounds that have different chemical and physical properties than they did before. • A chemical reaction is represented by writing a chemical equation – Using chemical formulas, symbols, and coefficients • An equation represents, the identities and relative amounts of what are called reactants and products in a chemical rxn – Reactants are the substances you start with in the reaction – Products are the results of the reaction
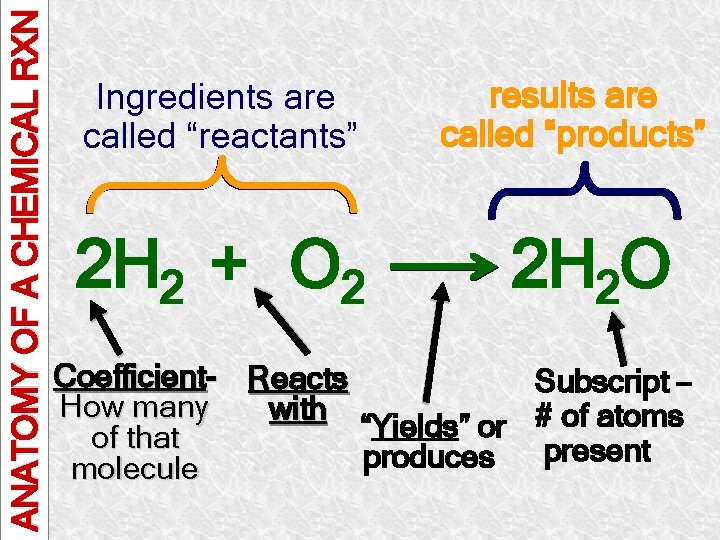
ANATOMY OF A CHEMICAL RXN Ingredients are called “reactants” 2 H 2 + O 2 results are called “products” 2 H 2 O Coefficient- Reacts Subscript – How many with # of atoms “Yields” or of that present produces molecule

INDICATIONS OF A CHEM RXN • To know for certain a chemical rxn has taken place requires evidence that 1 or more substances have changed identity • Absolute proof of such a change can only be provided by chemically analyzing the products. – However, certain observations can be made to provide qualitative indications of a successful chemical rxn. – Unexpected change

INDICATIONS OF A CHEM RXN 1) Production of a gas − Seeing gas bubbles produced when 2 substances are mixed is evidence of a reaction 2) Formation of a precipitate – If a solid appears after 2 solns are mixed, the solid is called a precipitate

INDICATIONS OF A CHEM RXN 3) Color change – Change in color is often an indication of a chemical reaction 4) Formation of heat and/or light – A release of energy as both heat & light is strong evidence of a chemical reaction
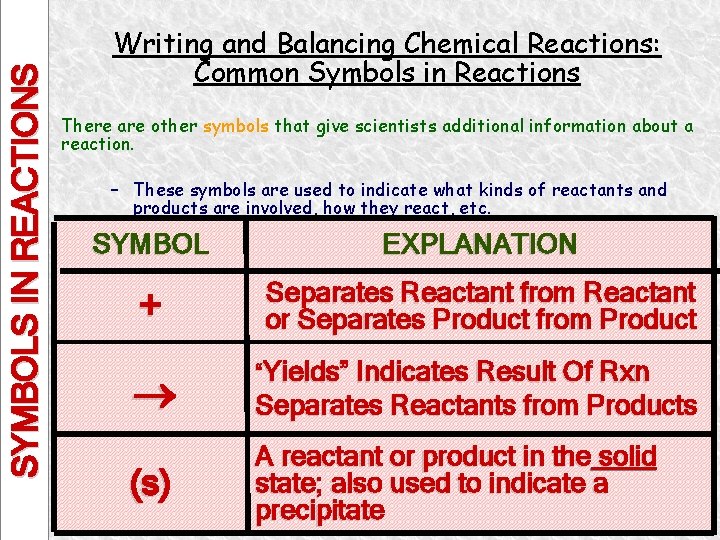
SYMBOLS IN REACTIONS Writing and Balancing Chemical Reactions: Common Symbols in Reactions There are other symbols that give scientists additional information about a reaction. – These symbols are used to indicate what kinds of reactants and products are involved, how they react, etc. SYMBOL EXPLANATION + Separates Reactant from Reactant or Separates Product from Product (s) “Yields” Indicates Result Of Rxn Separates Reactants from Products A reactant or product in the solid state; also used to indicate a precipitate
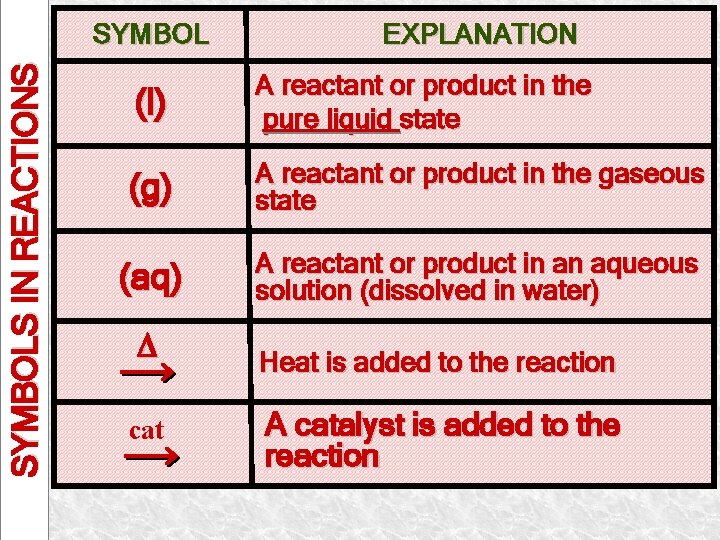
SYMBOLS IN REACTIONS SYMBOL EXPLANATION (l) A reactant or product in the pure liquid state (g) A reactant or product in the gaseous state (aq) A reactant or product in an aqueous solution (dissolved in water) cat Heat is added to the reaction A catalyst is added to the reaction
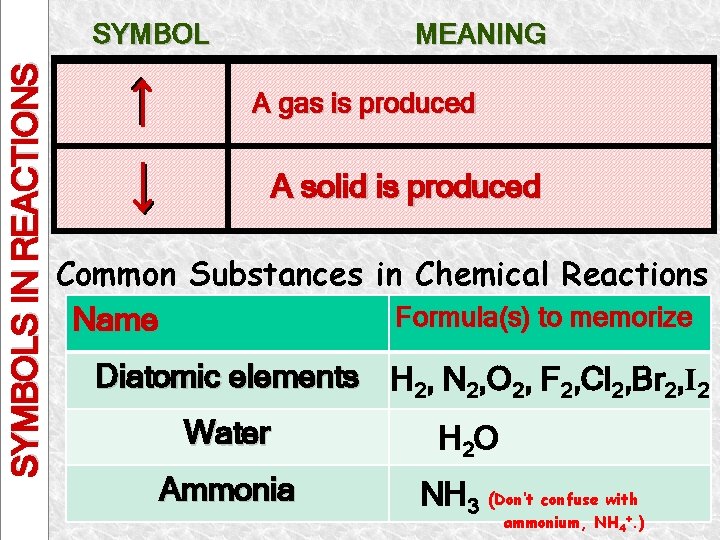
SYMBOLS IN REACTIONS SYMBOL MEANING A gas is produced A solid is produced Common Substances in Chemical Reactions Formula(s) to memorize Name Diatomic elements H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2 Water Ammonia H 2 O NH 3 (Don’t confuse with ammonium, NH 4+. )

Key Words Used in Describing Chemical Reactions Words used to separate reactants from other reactants. – “Reacts with” – “Mixed together” – “Bubbled through”

Key Words Used in Describing Chemical Reactions To separate reactants and products, you will generally see one of these words. *Yield *Burned *Produce *Decompose *Combusted *Give *Form
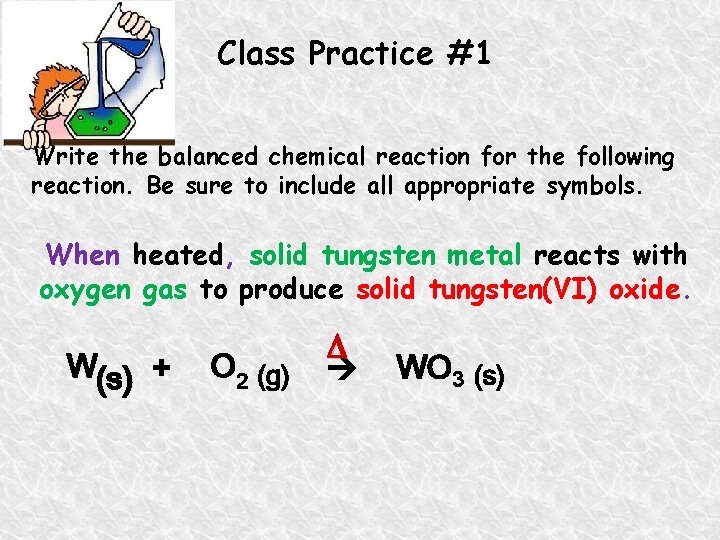
Class Practice #1 Write the balanced chemical reaction for the following reaction. Be sure to include all appropriate symbols. When heated, solid tungsten metal reacts with oxygen gas to produce solid tungsten(VI) oxide. W(s) + O 2 (g) WO 3 (s)
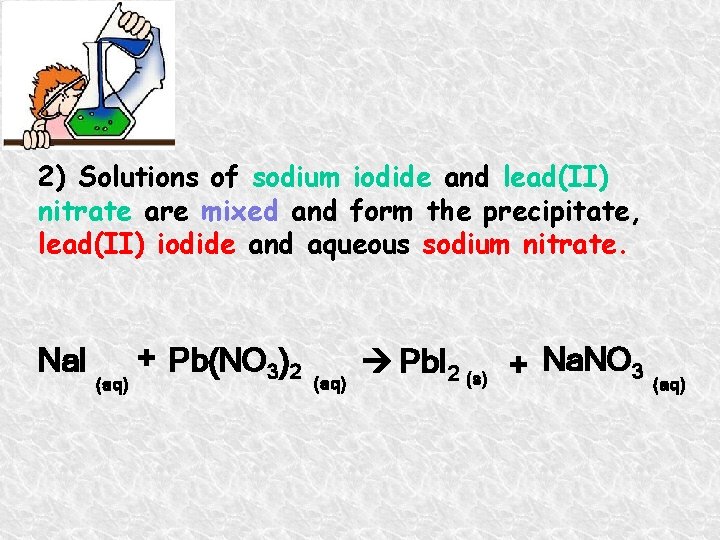
2) Solutions of sodium iodide and lead(II) nitrate are mixed and form the precipitate, lead(II) iodide and aqueous sodium nitrate. Na. I (aq) + Pb(NO 3)2 (aq) Pb. I 2 (s) + Na. NO 3 (aq)
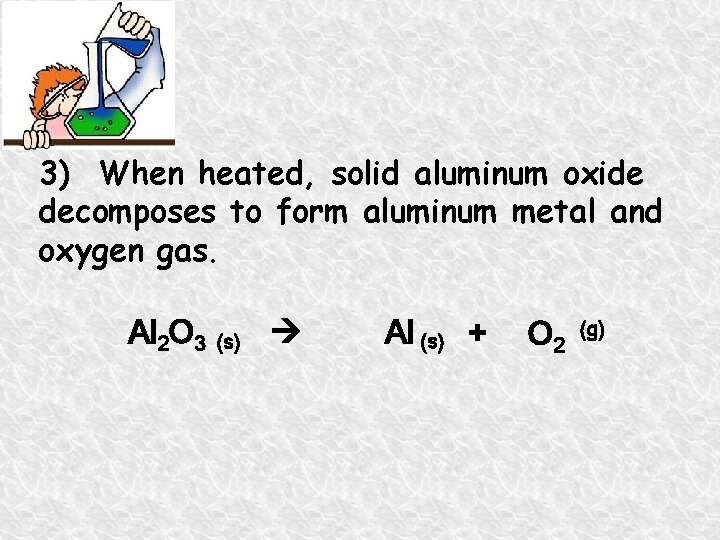
3) When heated, solid aluminum oxide decomposes to form aluminum metal and oxygen gas. Al 2 O 3 (s) Al (s) + O 2 (g)

Homework page 8

Day 3: Review…again (Page 9) • • • Cr 2 O 3 PO 2 Al. Cl 3 Ag. NO 3 S 2 O 5 ___________ ______

Father of Modern Chemistry 1743 - 1794 24 First Described the “Law of Conservation of Mass”
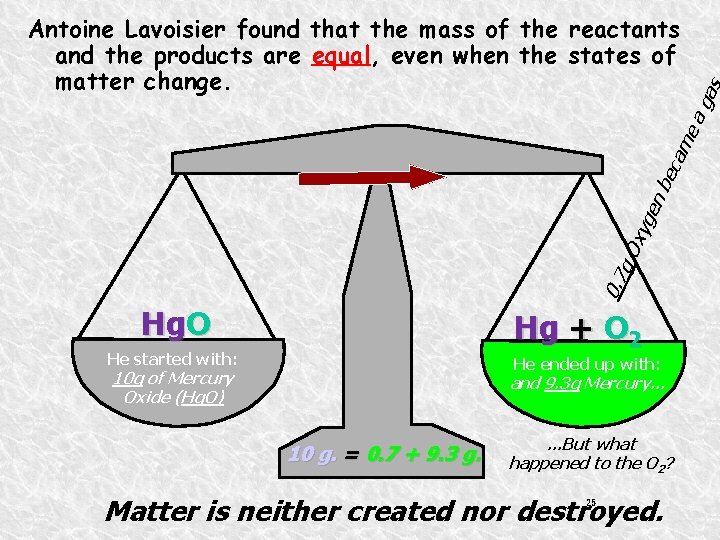
0. 7 g. O xyg en be s ga cam ea Antoine Lavoisier found that the mass of the reactants and the products are equal, even when the states of matter change. Hg. O Hg + O 2 He started with: He ended up with: 10 g of Mercury Oxide (Hg. O) and 9. 3 g Mercury… 10 g. = 0. 7 + 9. 3 g. . But what happened to the O 2? Matter is neither created nor destroyed. 25
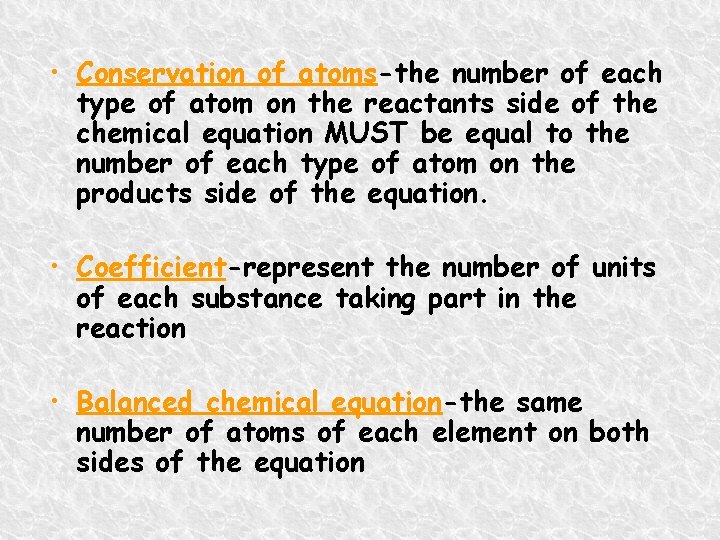
• Conservation of atoms-the number of each type of atom on the reactants side of the chemical equation MUST be equal to the number of each type of atom on the products side of the equation. • Coefficient-represent the number of units of each substance taking part in the reaction • Balanced chemical equation-the same number of atoms of each element on both sides of the equation

Four Steps to Balance Equations: 1. Set up your equation. 2. Count the number of atoms you have on both sides. 3. Balance by changing the coefficients and recounting. 4. Start the process again if it still does not balance. 27
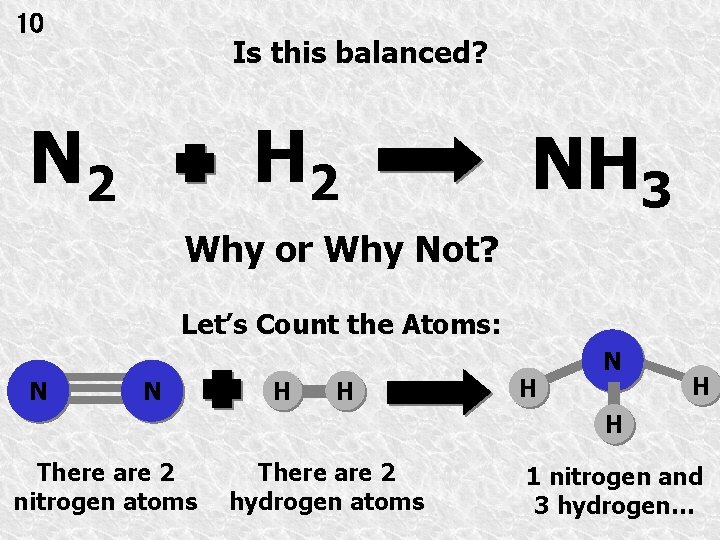
10 Is this balanced? H 2 NH 3 Why or Why Not? Let’s Count the Atoms: N N H H H N H H There are 2 nitrogen atoms There are 2 hydrogen atoms 1 nitrogen and 3 hydrogen…
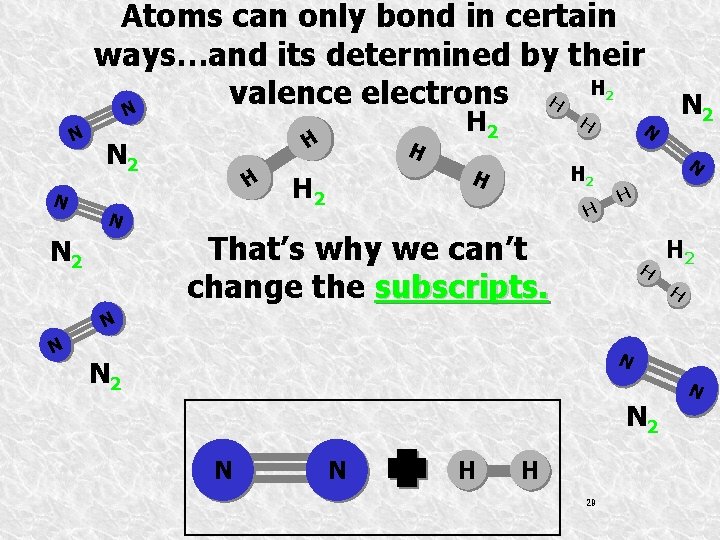
Atoms can only bond in certain ways…and its determined by their H 2 valence electrons H N N N H N 2 N N 2 H H H 2 H N 2 N N H That’s why we can’t change the subscripts. H H 2 H N N 2 N N H H 29 N
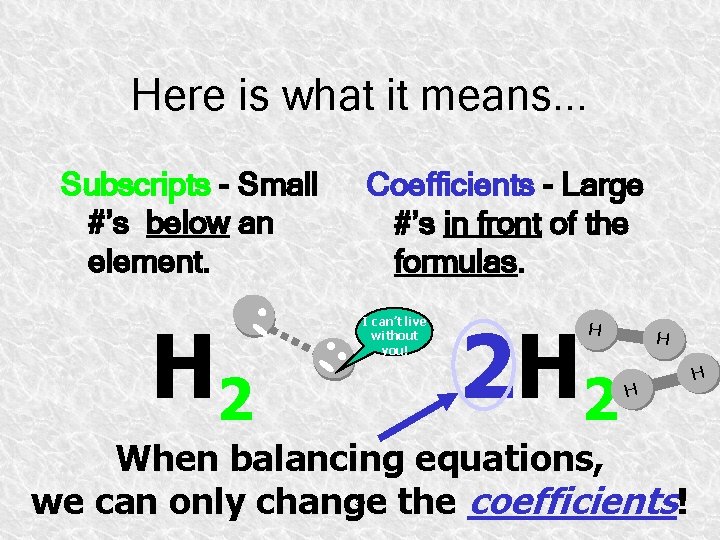
Here is what it means. . . Subscripts - Small #’s below an element. Coefficients - Large #’s in front of the formulas. H H 2 H I can’t live without you! 2 H 2 H H H When balancing equations, we can only change the coefficients! 30 H
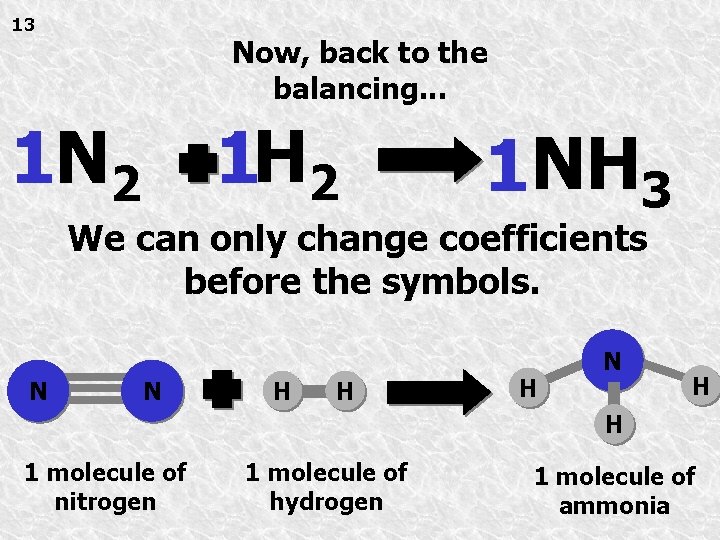
13 Now, back to the balancing. . . 1 H 2 1 NH 3 We can only change coefficients before the symbols. N N H H H N H H 1 molecule of nitrogen 1 molecule of hydrogen 1 molecule of ammonia
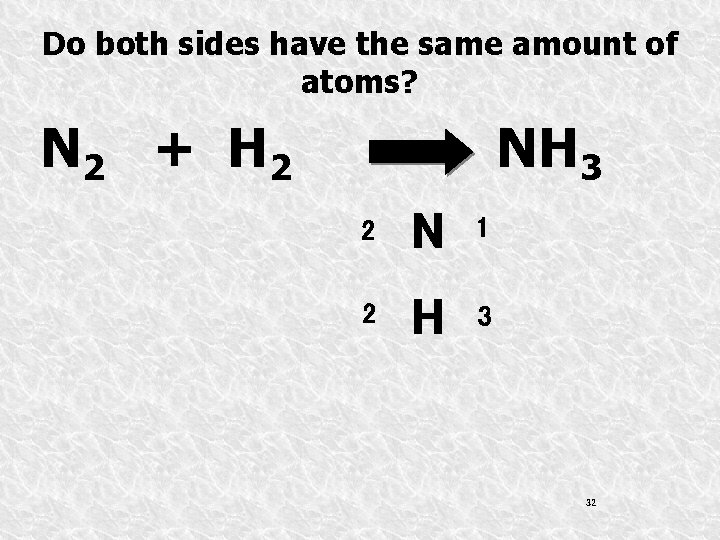
Do both sides have the same amount of atoms? N 2 + H 2 NH 3 2 N 1 2 H 3 32
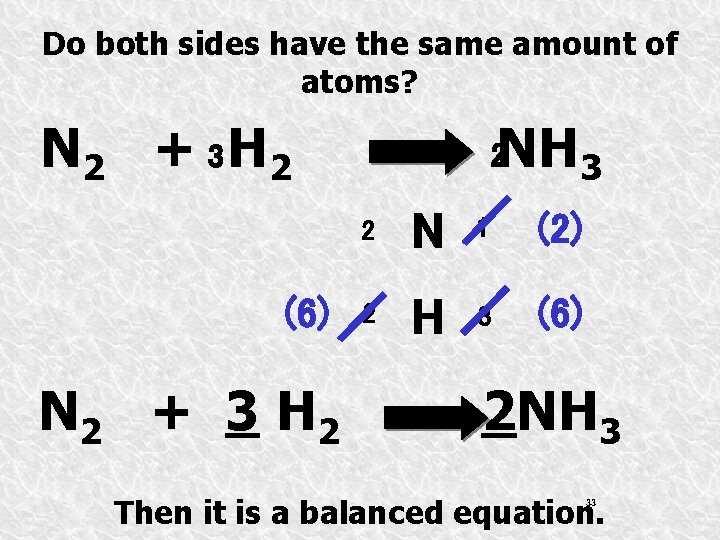
Do both sides have the same amount of atoms? N 2 + 3 H 2 (6) N 2 + 3 H 2 NH 3 2 2 N 1 (2) 2 H 3 (6) 2 NH 3 Then it is a balanced equation. 33
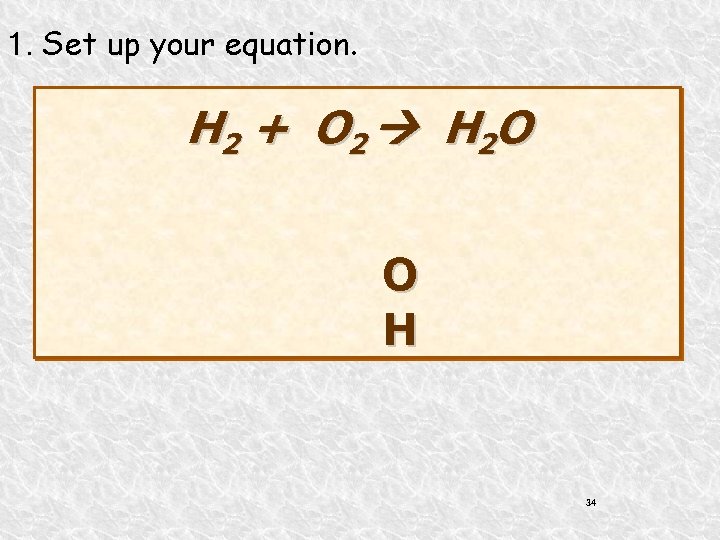
1. Set up your equation. H 2 + O 2 H 2 O O H 34

2. Count the number of atoms you have of each on both sides. H 2 + O 2 H 2 O 1 2 H 2 35
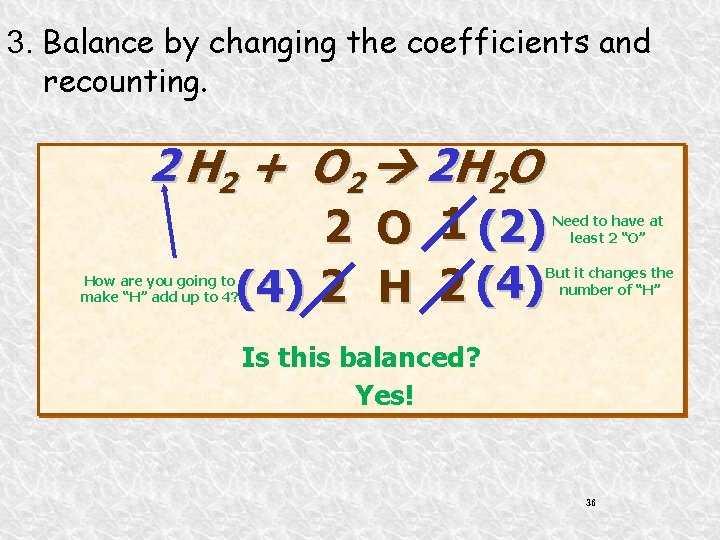
3. Balance by changing the coefficients and recounting. 2 H 2 + O 2 2 H 2 O 1 (2) (4) 2 H 2 (4) Need to have at least 2 “O” But it changes the number of “H” How are you going to make “H” add up to 4? Is this balanced? Yes! 36
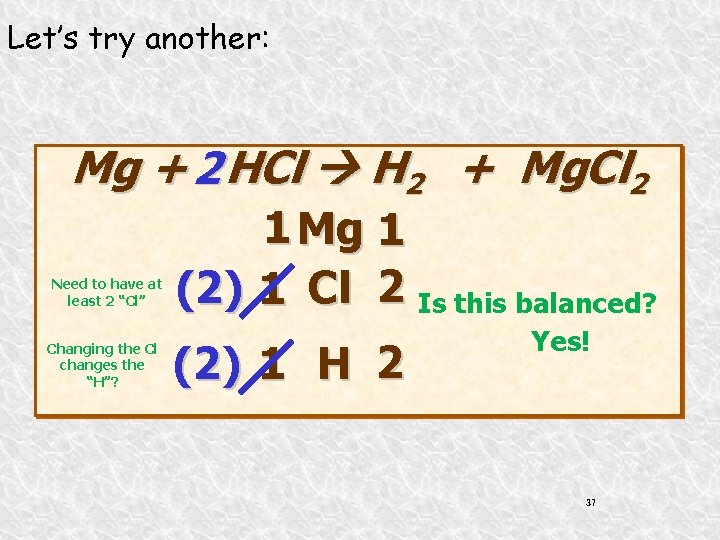
Let’s try another: Mg + 2 HCl H 2 + Mg. Cl 2 Need to have at least 2 “Cl” Changing the Cl changes the “H”? 1 Mg 1 (2) 1 Cl 2 Is this balanced? (2) 1 H 2 Yes! 37
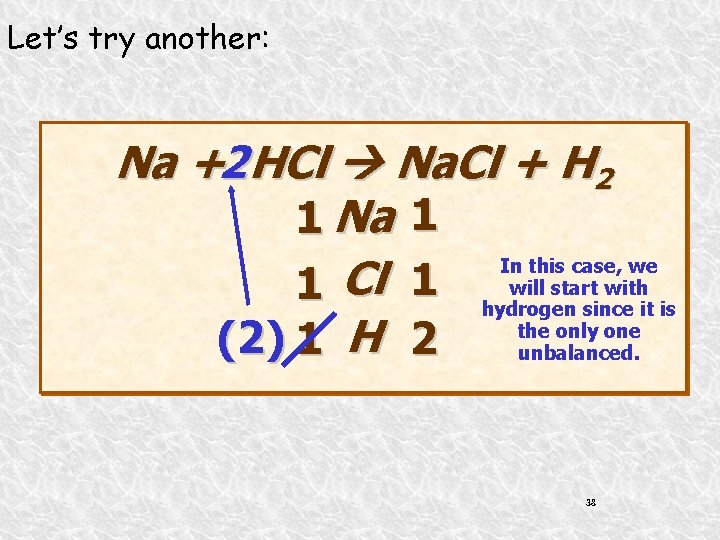
Let’s try another: Na +2 HCl Na. Cl + H 2 1 Na 1 In this case, we will start with 1 Cl 1 hydrogen since it is the only one H (2) 1 2 unbalanced. 38
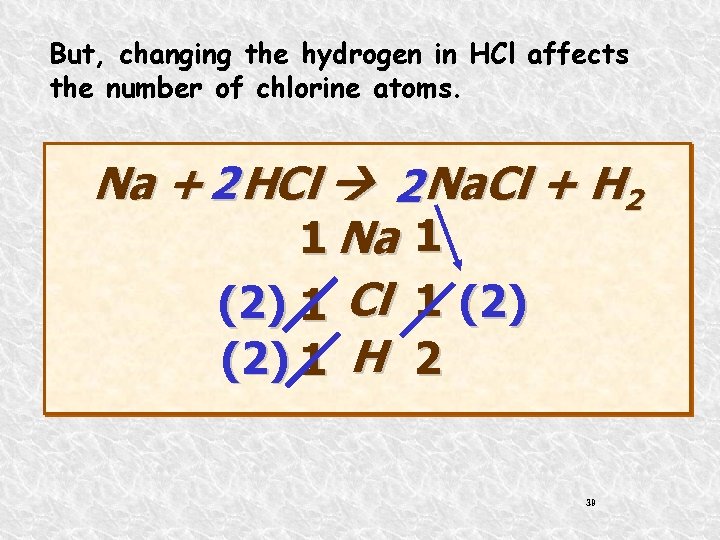
But, changing the hydrogen in HCl affects the number of chlorine atoms. Na + 2 HCl 2 Na. Cl + H 2 1 Na 1 (2) 1 Cl 1 (2) 1 H 2 39
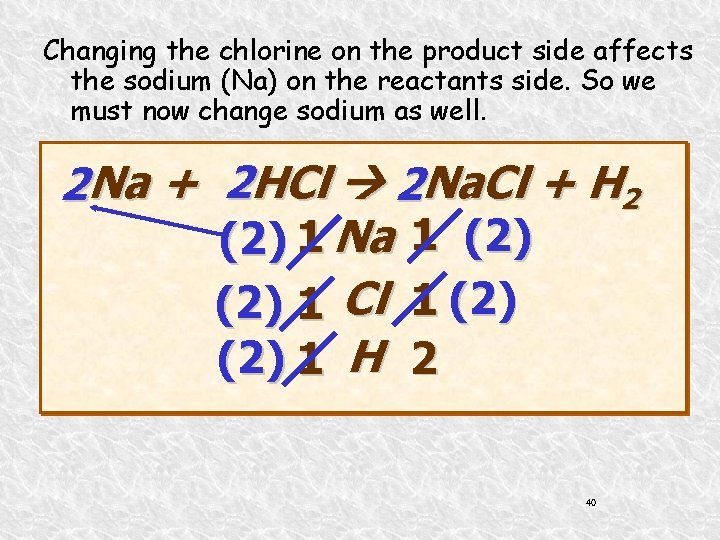
Changing the chlorine on the product side affects the sodium (Na) on the reactants side. So we must now change sodium as well. 2 Na + 2 HCl 2 Na. Cl + H 2 (2) 1 Na 1 (2) 1 Cl 1 (2) 1 H 2 40

Homework : Pg 10 and 11
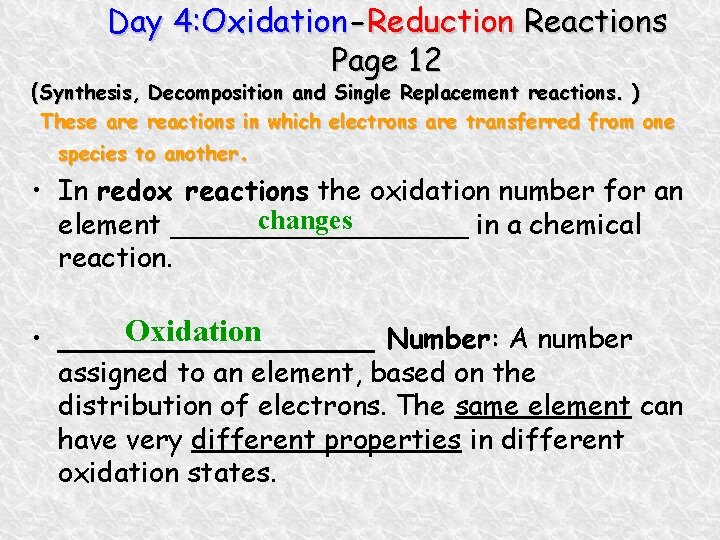
Day 4: Oxidation-Reduction Reactions Page 12 (Synthesis, Decomposition and Single Replacement reactions. ) These are reactions in which electrons are transferred from one species to another. • In redox reactions the oxidation number for an changes element _________ in a chemical reaction. Oxidation • _________ Number: A number assigned to an element, based on the distribution of electrons. The same element can have very different properties in different oxidation states.
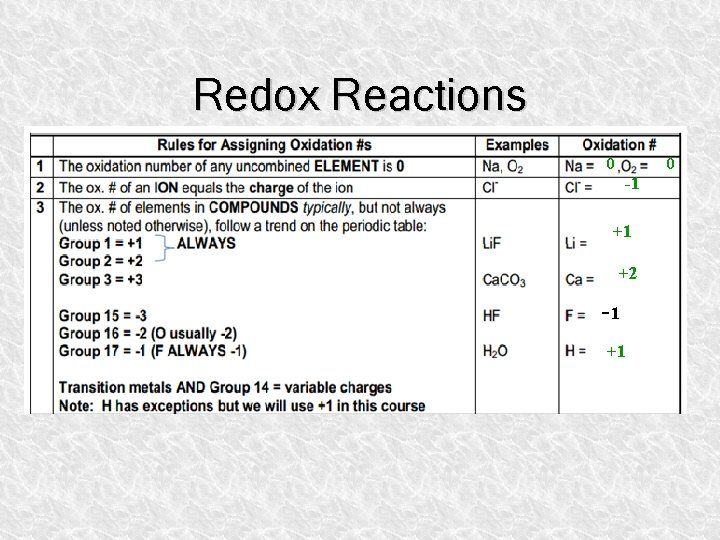
Redox Reactions 0 0 -1 +1 +2 -1 +1
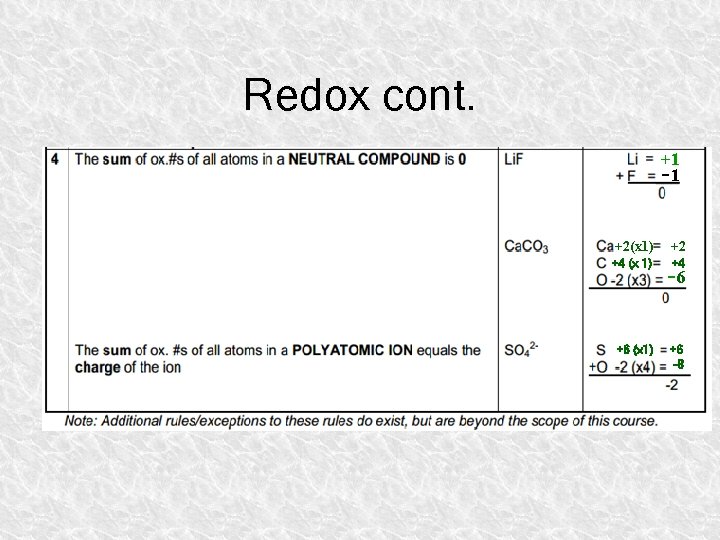
Redox cont. +1 -1 +2(x 1) +2 +4 (x 1) +4 -6 +6 (x 1) +6 -8
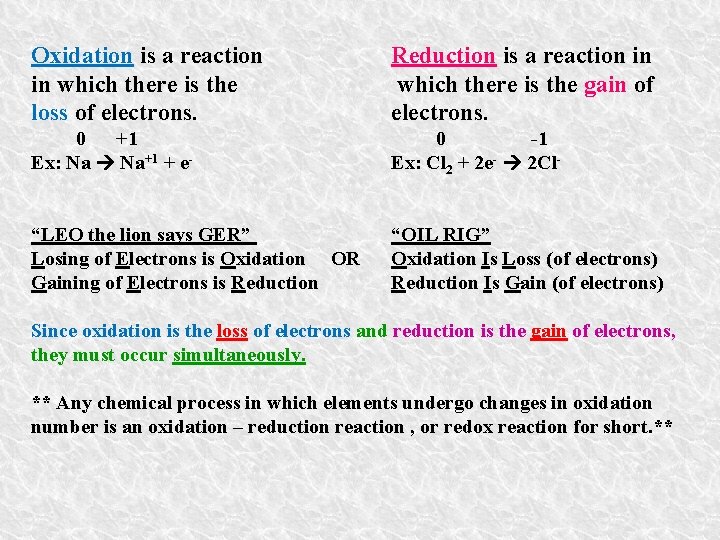
Oxidation is a reaction in which there is the loss of electrons. Reduction is a reaction in which there is the gain of electrons. 0 +1 Ex: Na Na+1 + e- 0 -1 Ex: Cl 2 + 2 e- 2 Cl- “LEO the lion says GER” Losing of Electrons is Oxidation OR Gaining of Electrons is Reduction “OIL RIG” Oxidation Is Loss (of electrons) Reduction Is Gain (of electrons) Since oxidation is the loss of electrons and reduction is the gain of electrons, they must occur simultaneously. ** Any chemical process in which elements undergo changes in oxidation number is an oxidation – reduction reaction , or redox reaction for short. **
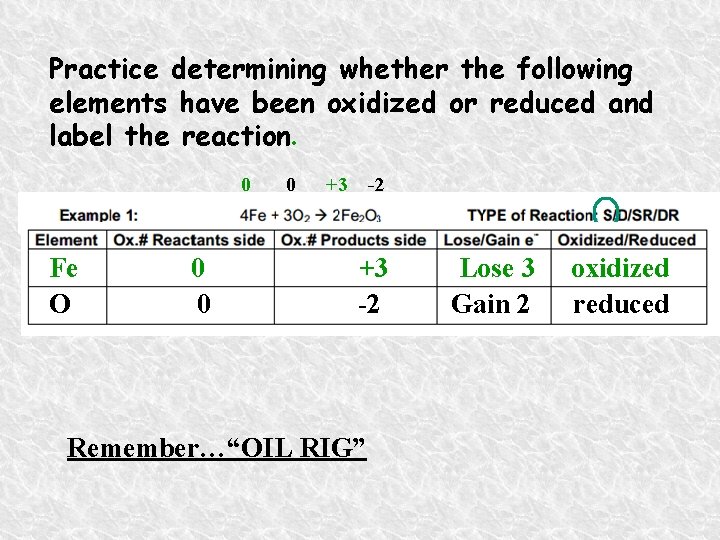
Practice determining whether the following elements have been oxidized or reduced and label the reaction. 0 Fe O 0 0 0 +3 -2 Remember…“OIL RIG” Lose 3 Gain 2 oxidized reduced
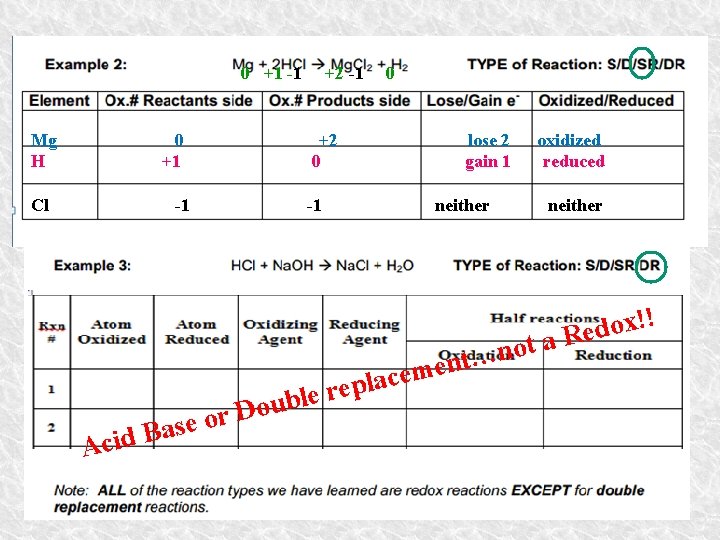
0 +1 -1 Mg H 0 +1 Cl +2 0 -1 Aci or e s a d. B +2 -1 -1 D l p e r ouble 0 lose 2 gain 1 oxidized reduced neither t o n … t n e m e ac neither ! ! x o d a Re

Now its YOUR TURN…. Homework!! Page 14

Day 5: Types of Redox Reactions Page 15
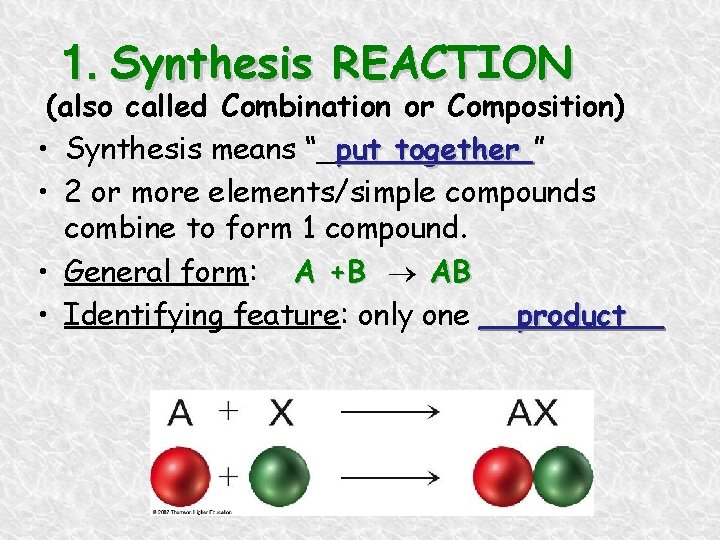
1. Synthesis REACTION (also called Combination or Composition) • Synthesis means “_put together ” • 2 or more elements/simple compounds combine to form 1 compound. • General form: A +B AB • Identifying feature: only one __product__
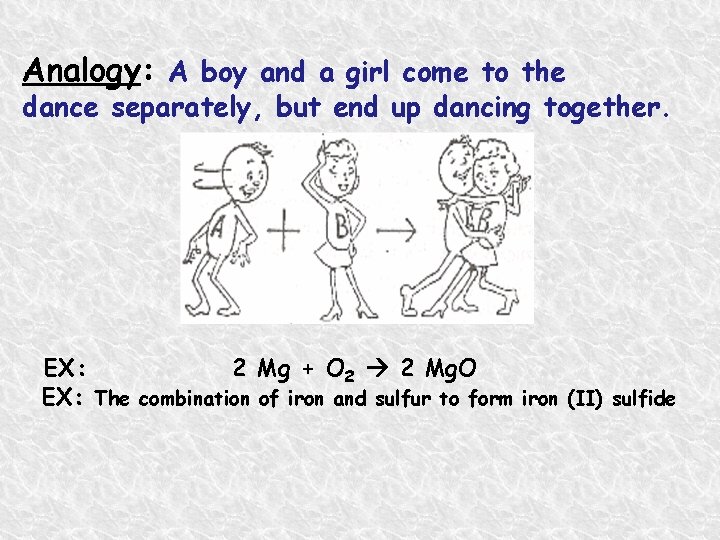
Analogy: A boy and a girl come to the dance separately, but end up dancing together. EX: 2 Mg + O 2 2 Mg. O EX: The combination of iron and sulfur to form iron (II) sulfide

EX: Burning charcoal C (s) + O 2 (g) → CO 2 (g) Fig. 8 -11, p. 214
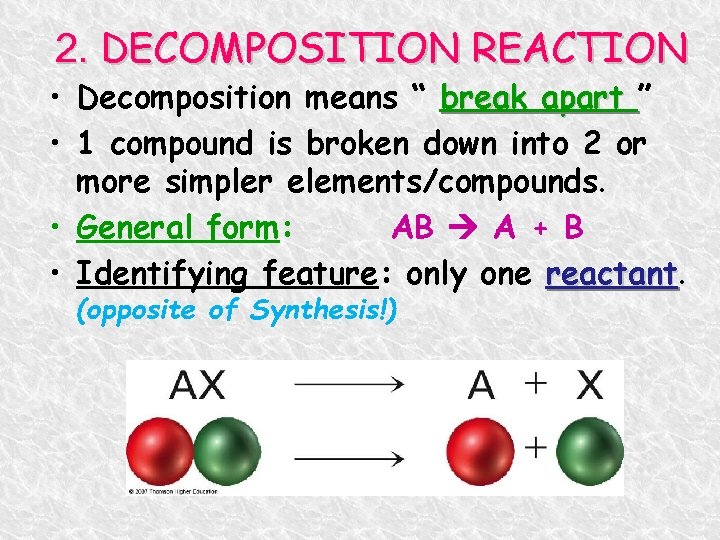
2. DECOMPOSITION REACTION • Decomposition means “ break apart ” • 1 compound is broken down into 2 or more simpler elements/compounds. • General form: AB A + B • Identifying feature: only one reactant (opposite of Synthesis!)
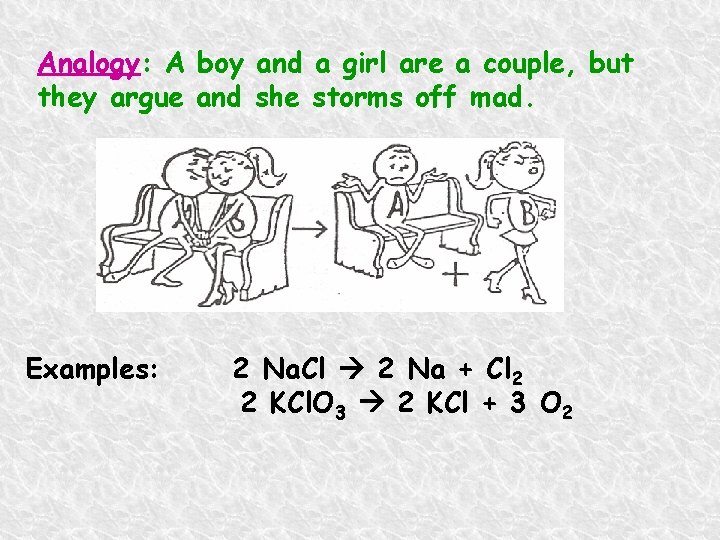
Analogy: A boy and a girl are a couple, but they argue and she storms off mad. Examples: 2 Na. Cl 2 Na + Cl 2 2 KCl. O 3 2 KCl + 3 O 2
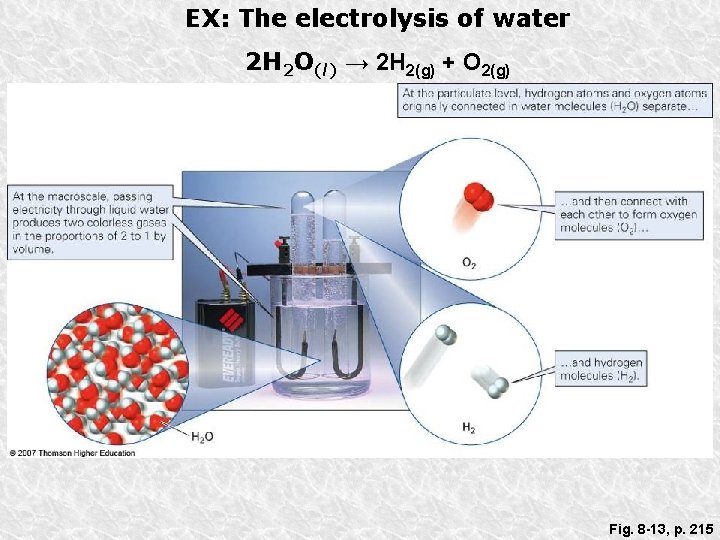
EX: The electrolysis of water 2 H 2 O(l) → 2 H 2(g) + O 2(g) Fig. 8 -13, p. 215
3. Combustion Also known as _burning_. Always follows the same form: Compound containing C and H (& sometimes O) + O 2 CO 2 + H 2 O Note: In a combustion reaction, the compound always burns in oxygen gas and always releases carbon dioxide and water.
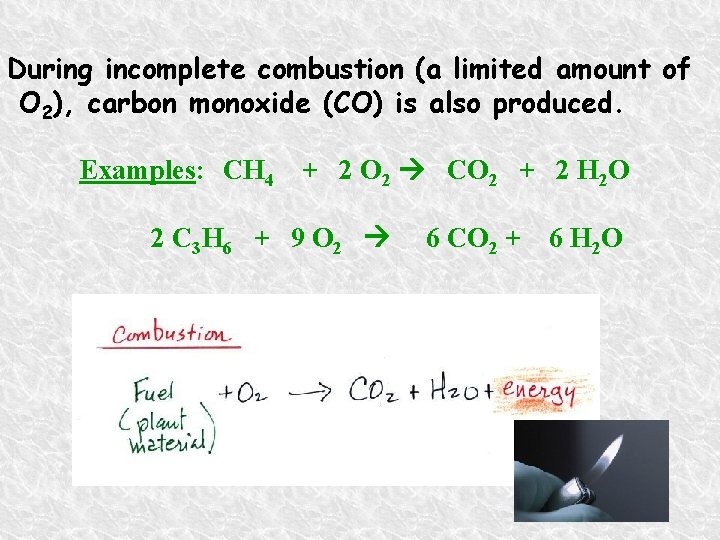
During incomplete combustion (a limited amount of O 2), carbon monoxide (CO) is also produced. Examples: CH 4 + 2 O 2 CO 2 + 2 H 2 O 2 C 3 H 6 + 9 O 2 6 CO 2 + 6 H 2 O
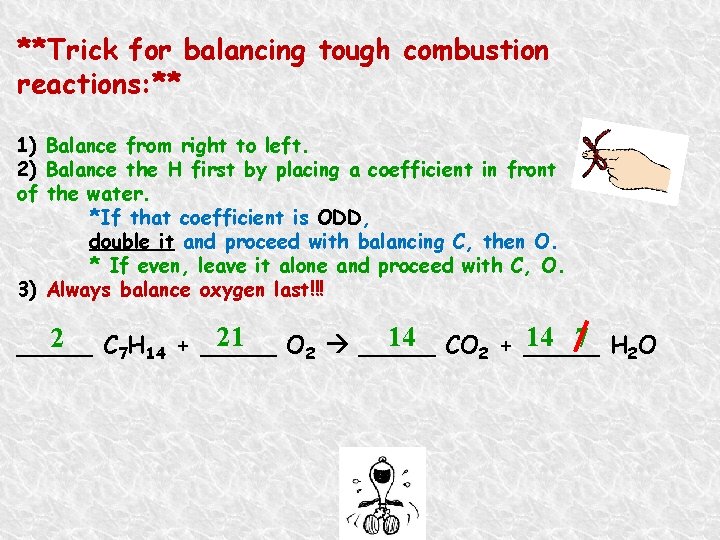
**Trick for balancing tough combustion reactions: ** 1) Balance from right to left. 2) Balance the H first by placing a coefficient in front of the water. *If that coefficient is ODD, double it and proceed with balancing C, then O. * If even, leave it alone and proceed with C, O. 3) Always balance oxygen last!!! 21 O 2 _____ 14 CO 2 + _____ 14 7 H 2 O 2 C 7 H 14 + _____
Homework pg 17

Day 6: Redox continued… Single Replacement or Displacement Reactions
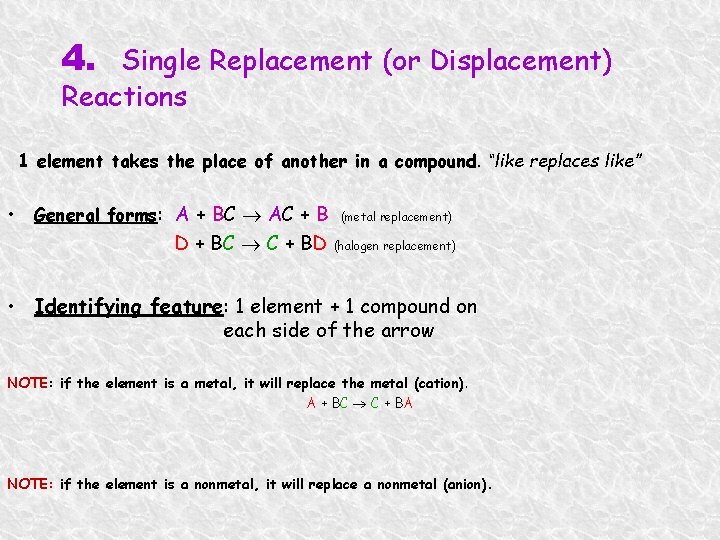
4. Single Replacement (or Displacement) Reactions 1 element takes the place of another in a compound. “like replaces like” • General forms: A + BC AC + B (metal replacement) D + BC C + BD (halogen replacement) • Identifying feature: 1 element + 1 compound on each side of the arrow NOTE: if the element is a metal, it will replace the metal (cation). A + BC C + BA NOTE: if the element is a nonmetal, it will replace a nonmetal (anion).
Analogy: A boy and a girl are dancing, but then another boy “cuts in” and dances with the girl, leaving the first boy alone. Or a boy and a girl are dancing, but then another girl “cuts in” and dances with the boy, leaving the first girl alone. “Like” must replace “like” Examples: Metal replacement: 2 Na + Cu. Cl 2 2 Na. Cl + Cu Halogen replacement: F 2 + 2 KCl 2 KF + Cl 2

BUT the boy/girl will not always be able to “cut in. ” Sometimes the other boy/girl will not let them! Rules for Reactions Including: Metals with Metals / Metals with Acids We must use the activity series to predict whether or not the replacement will occur. If an element is more reactive (found higher up in the activity series) than another element, it WILL replace that element. (Higher element will only replace something lower, not lower to higher) Halogen Activity Series (same order as on Periodic Table) F Cl Br I (most reactive) (least reactive) Examples: no Can Al replace Li? ____ yes Can Cu replace Au? ____ yes Can Br replace I? ____ no Can Cl replace F? ____ **Note: This activity series is only used for single replacement reactions.
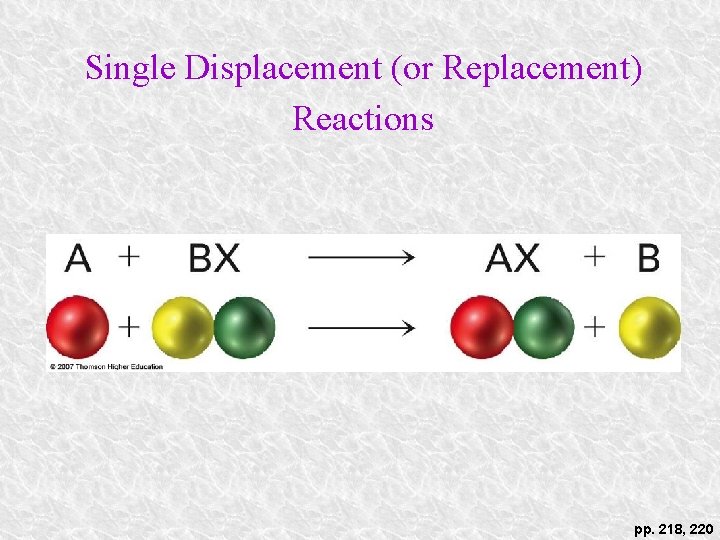
Single Displacement (or Replacement) Reactions pp. 218, 220
Predicting Single Replacement Reactions • Must use the activity series • Remember a metal can only replace another metal or hydrogen
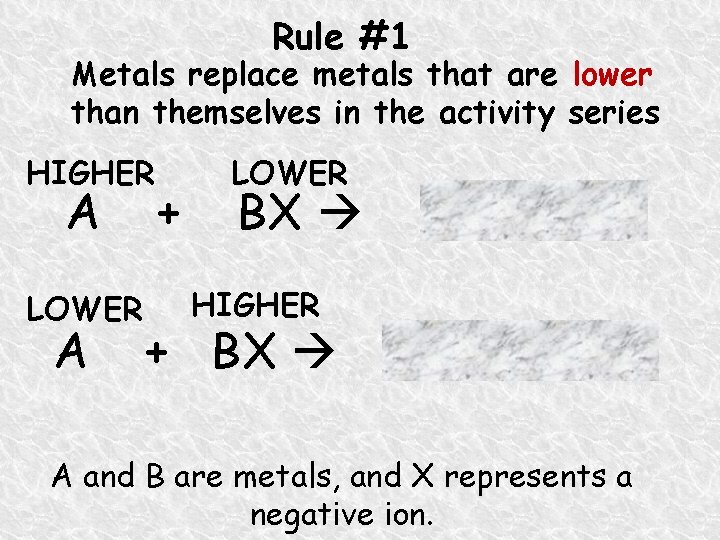
Rule #1 Metals replace metals that are lower than themselves in the activity series HIGHER A LOWER A + LOWER BX B + AX HIGHER + BX no reaction A and B are metals, and X represents a negative ion.
Examples Which reactions will occur? 1. Mg(s) + Al. Cl 3(aq) Yes, the metal in the compound can trade up!

Fe. Cl 2(aq) + Cu(s) NR Sodium + Lithium Chloride NR Silver Nitrate + Zinc Yes
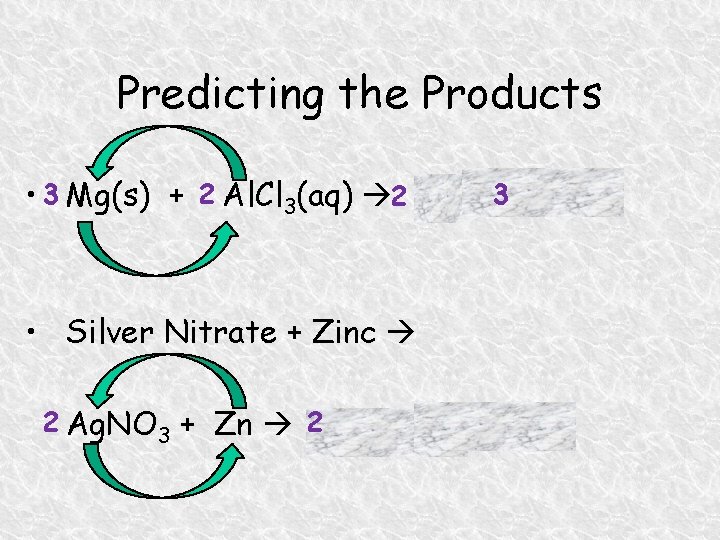
Predicting the Products • 3 Mg(s) + 2 Al. Cl 3(aq) 2 Al + 3 Mg. Cl 2 • Silver Nitrate + Zinc 2 Ag. NO 3 + Zn 2 Ag + Zn(NO 3)2
![Rule #2 Any metal above [H 2] will react with an acid to produce Rule #2 Any metal above [H 2] will react with an acid to produce](http://slidetodoc.com/presentation_image_h2/8a666cae2a39d286df890a385f63e532/image-70.jpg)
Rule #2 Any metal above [H 2] will react with an acid to produce a compound + H 2. *Put H 2 at the end of your arrow* Example: M + HX MX + H 2 M = metal X = negative ion
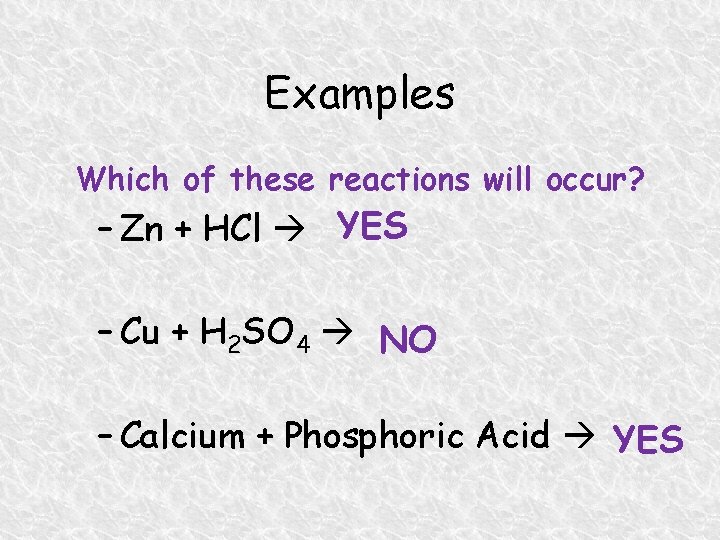
Examples Which of these reactions will occur? – Zn + HCl YES – Cu + H 2 SO 4 NO – Calcium + Phosphoric Acid YES
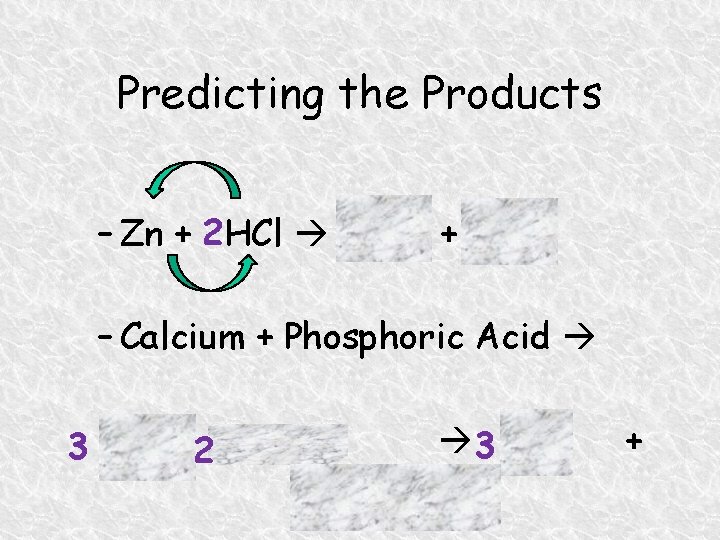
Predicting the Products – Zn + 2 HCl Zn. Cl 2 + H 2 – Calcium + Phosphoric Acid 3 H 2 3 Ca +2 H 3 PO 4 Ca 3(PO 4)2 +

Homework Page 21

Day 7: Redox continued… Single Replacement Page 22
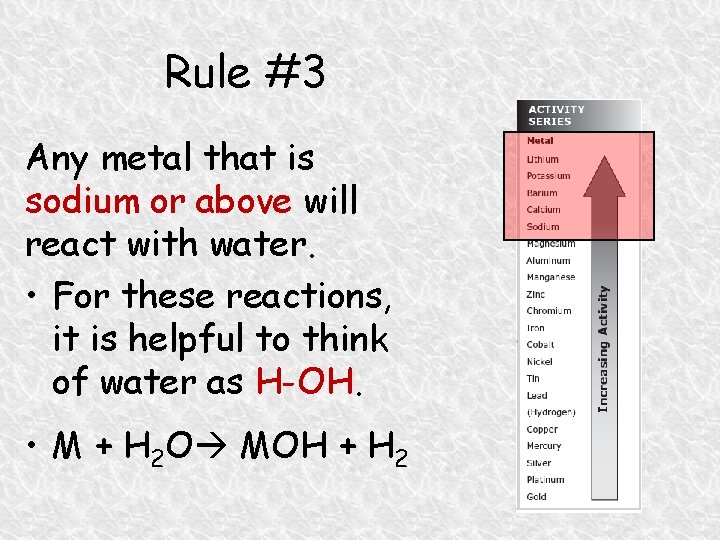
Rule #3 Any metal that is sodium or above will react with water. • For these reactions, it is helpful to think of water as H-OH. • M + H 2 O MOH + H 2

Will these reactions occur? • Na + H 2 O YES • Ca in water. YES • Cd + H 2 O NO
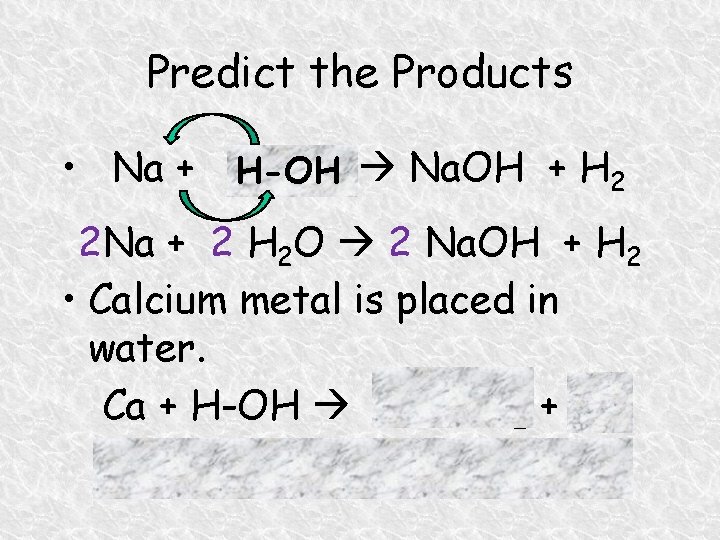
Predict the Products • Na + H Na. OH + H 2 H-OH 2 O 2 Na + 2 H 2 O 2 Na. OH + H 2 • Calcium metal is placed in water. Ca + H-OH Ca(OH)2 + H 2 Ca + 2 H 2 O Ca(OH)2 + H 2
Rule #4: Halogens will only replace other halogens that are higher in the Activity Series. Halogens will NOT replace metals!!! • There is an activity series for the halogens. It is the group on your periodic table.
Examples Will these reactions occur? • F 2 + Al. Cl 3 Yes • Chlorine gas is bubbled through a solution of sodium Yes iodide. • Br 2 + KCl No
Predict the Products • 3 F 2 + 2 Al. Cl 3 3 Cl 2 + 2 Al. F 3 • Chlorine gas is bubbled through a solution of sodium iodide. Cl 2 + 2 Na. I I 2 + 2 Na. Cl

Experiment of the Types of Reactions (Chalk Lab) • You will be using a Bunsen burner, hair must be pulled back and no sleeves. • The chalk will be hot, do not touch! • Please put all waste in the waste container, NOT DOWN THE SINK!

Day 7 HW pg 24.

Day 8 Precipitate or Double Replacement Reactions Understanding solubility! Page 25
Double Replacement (or Displacement) Reactions Ions from 2 ionic compounds switch places. It is easier to simply remember that the cations (+ ions) exchange anions (- ions). General form: AB + CD CB + AD Combine the inner ions and outer ions. Identifying feature: 2 compounds on each side of the equation
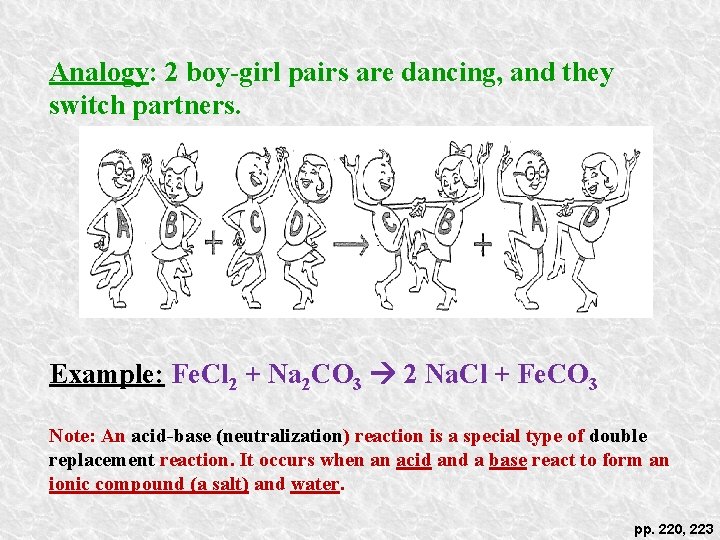
Analogy: 2 boy-girl pairs are dancing, and they switch partners. Example: Fe. Cl 2 + Na 2 CO 3 2 Na. Cl + Fe. CO 3 Note: An acid-base (neutralization) reaction is a special type of double replacement reaction. It occurs when an acid and a base react to form an ionic compound (a salt) and water. pp. 220, 223

Soluble versus Insoluble • A Soluble Compound… – Will dissolve. – Will be labeled as aqueous or (aq) in a chemical reaction. • An Insoluble Compound… – Will NOT dissolve. – Will be labeled as a solid or (s) in a chemical reaction.
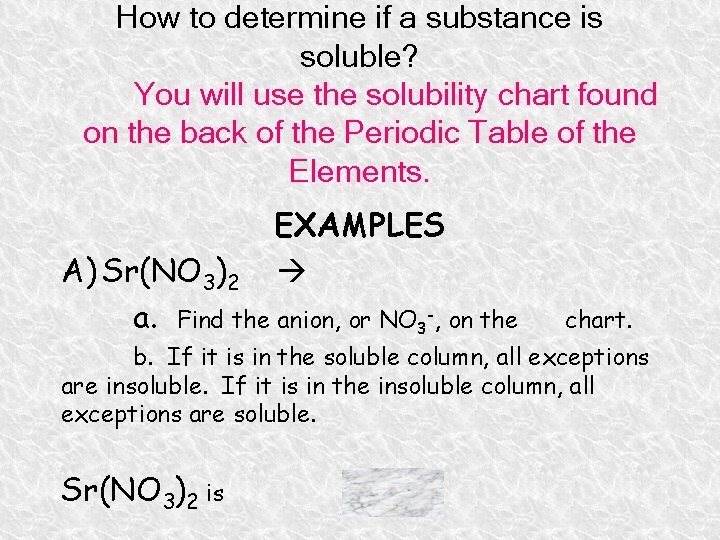
How to determine if a substance is soluble? You will use the solubility chart found on the back of the Periodic Table of the Elements. EXAMPLES A) Sr(NO 3)2 a. Find the anion, or NO 3 -, on the chart. b. If it is in the soluble column, all exceptions are insoluble. If it is in the insoluble column, all exceptions are soluble. Sr(NO 3)2 is soluble
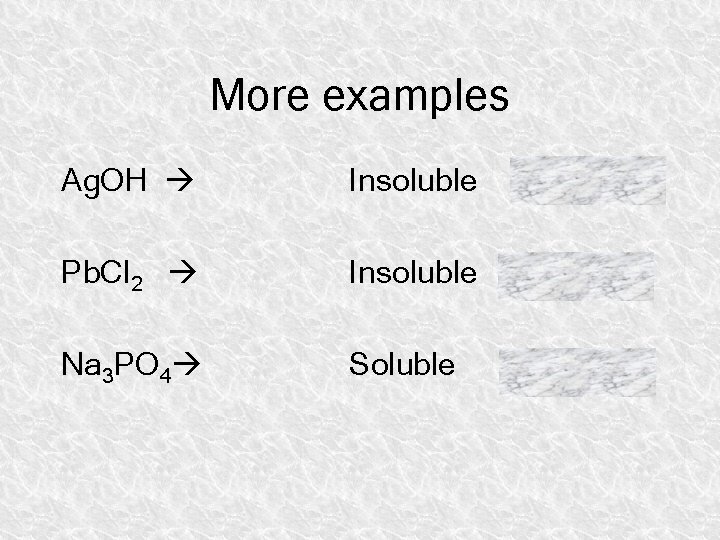
More examples Ag. OH Insoluble Pb. Cl 2 Insoluble Na 3 PO 4 Soluble
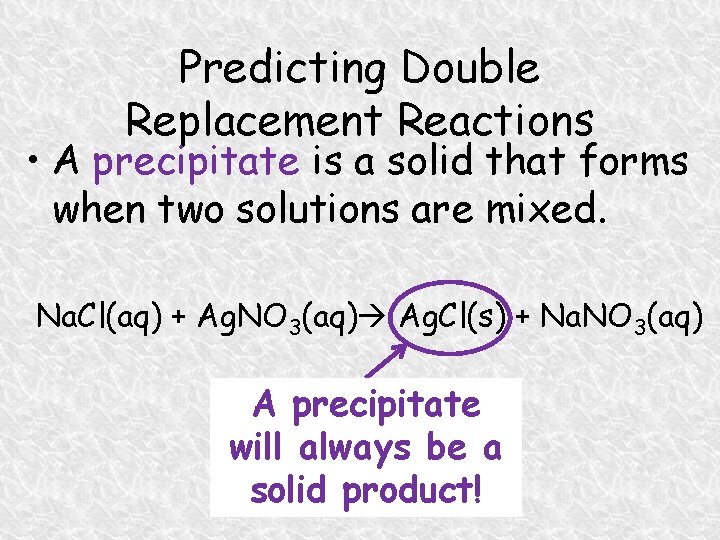
Predicting Double Replacement Reactions • A precipitate is a solid that forms when two solutions are mixed. Na. Cl(aq) + Ag. NO 3(aq) Ag. Cl(s) + Na. NO 3(aq) A precipitate will always be a solid product!

Na. Cl(aq) + Ag. NO 3(aq) → Na. NO 3(aq) + Ag. Cl(s) Fig. 8 -18, p. 221

Predicting Double Replacement Reactions AX + BY + AX Your positive ions should switch places! Cu. Cl 2(aq) + Na 3 PO 4(aq) Na. Cl(aq) +Cu 3(PO 4)2 (s) Cu and Na will switch places! But does a precipitate form? YES!
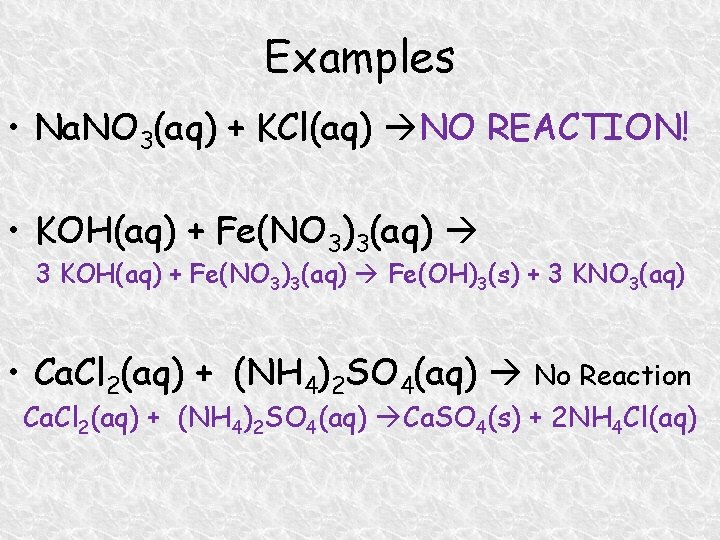
Examples • Na. NO 3(aq) + KCl(aq) NO REACTION! • KOH(aq) + Fe(NO 3)3(aq) 3 KOH(aq) + Fe(NO 3)3(aq) Fe(OH)3(s) + 3 KNO 3(aq) • Ca. Cl 2(aq) + (NH 4)2 SO 4(aq) No Reaction Ca. Cl 2(aq) + (NH 4)2 SO 4(aq) Ca. SO 4(s) + 2 NH 4 Cl(aq)

Day 8 HW page 27.
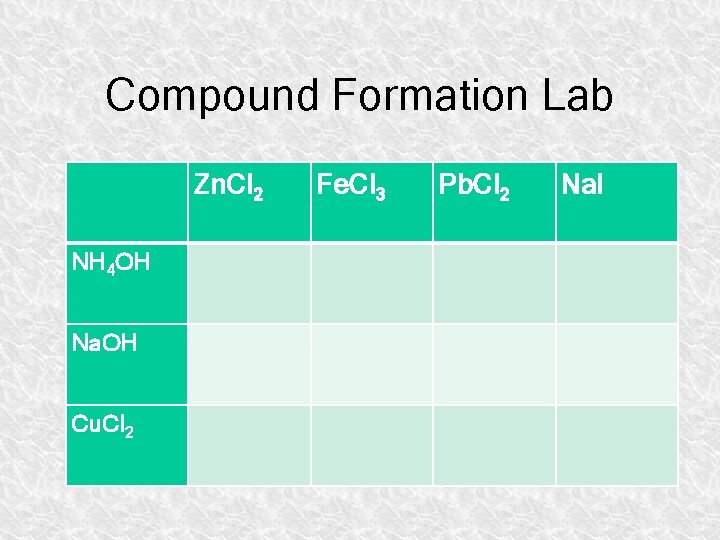
Compound Formation Lab Zn. Cl 2 NH 4 OH Na. OH Cu. Cl 2 Fe. Cl 3 Pb. Cl 2 Na. I
- Slides: 94