Unit 8 Chemical Reactions Part 2 Predicting Products





- Slides: 24

Unit #8 Chemical Reactions Part 2 Predicting Products

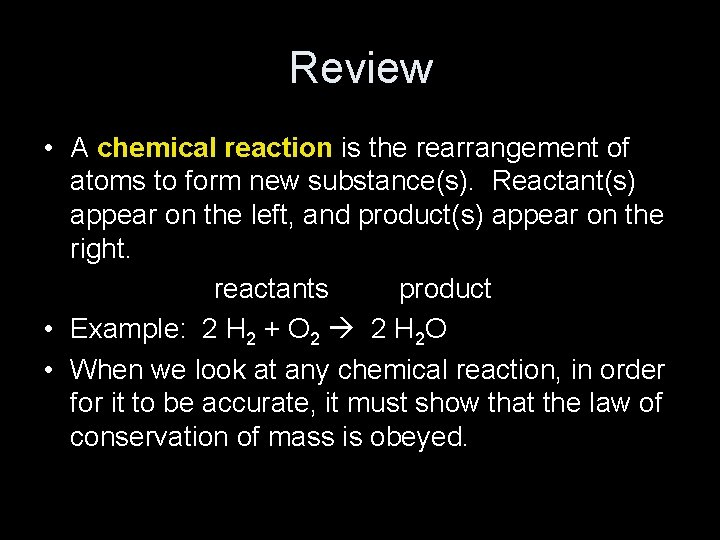
Review • A chemical reaction is the rearrangement of atoms to form new substance(s). Reactant(s) appear on the left, and product(s) appear on the right. reactants product • Example: 2 H 2 + O 2 2 H 2 O • When we look at any chemical reaction, in order for it to be accurate, it must show that the law of conservation of mass is obeyed.

Law of Conservation of Mass • mass is neither created nor destroyed in a chemical reaction • total mass stays the same • atoms can only rearrange • So, we need to make sure that there are the same number of each type of atom on each side of the chemical equation. • To do this, we add coefficients in front of the compounds until these atoms are “balanced. ”

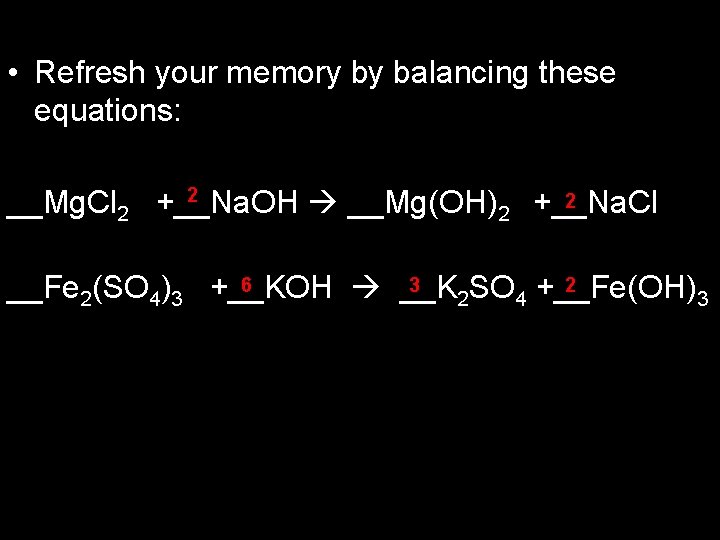
• Refresh your memory by balancing these equations: 2 2 __Mg. Cl 2 +__Na. OH __Mg(OH)2 +__Na. Cl 6 3 2 __Fe 2(SO 4)3 +__KOH __K SO +__Fe(OH) 2 4 3

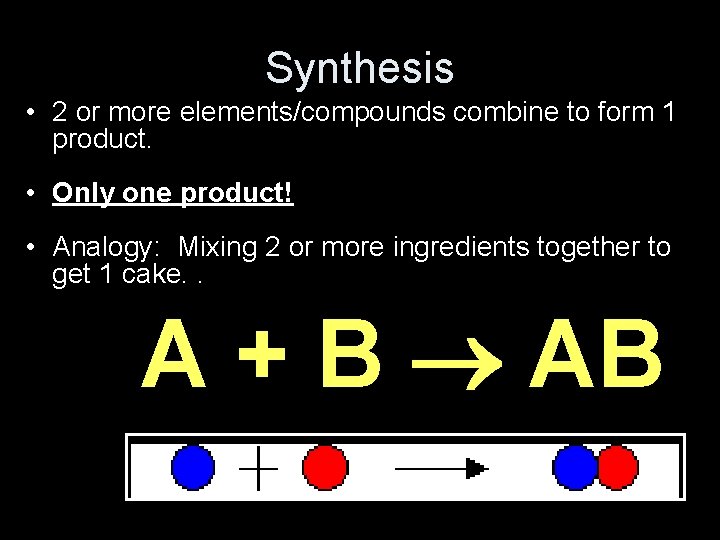
Synthesis • 2 or more elements/compounds combine to form 1 product. • Only one product! • Analogy: Mixing 2 or more ingredients together to get 1 cake. . A + B AB

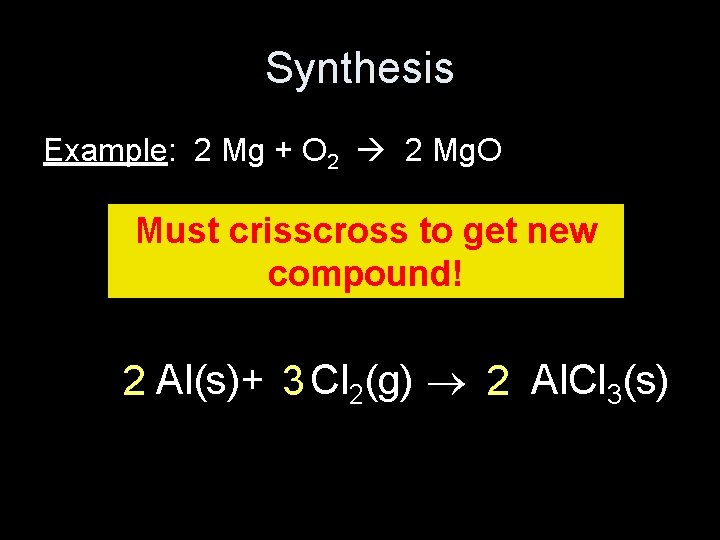
Synthesis Example: 2 Mg + O 2 2 Mg. O Must crisscross to get new compound! 2 Al(s)+ 3 Cl 2(g) 2 Al. Cl 3(s)

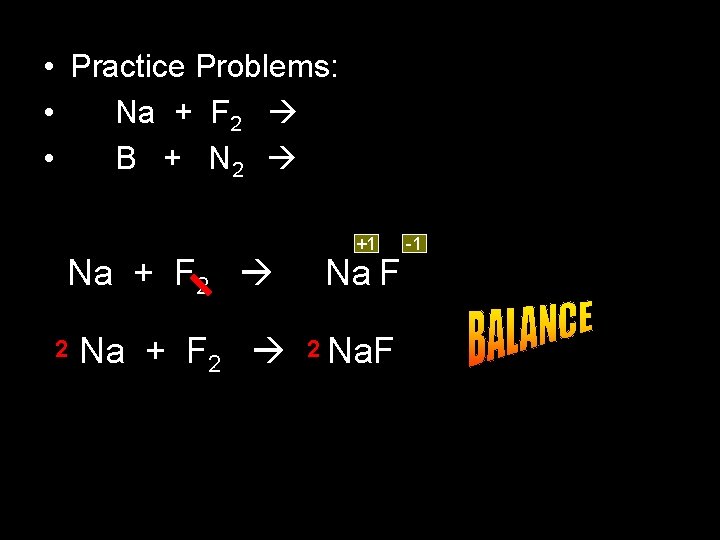
• Practice Problems: • Na + F 2 • B + N 2 Na + F 2 2 Na + F 2 +1 Na F 2 Na. F -1

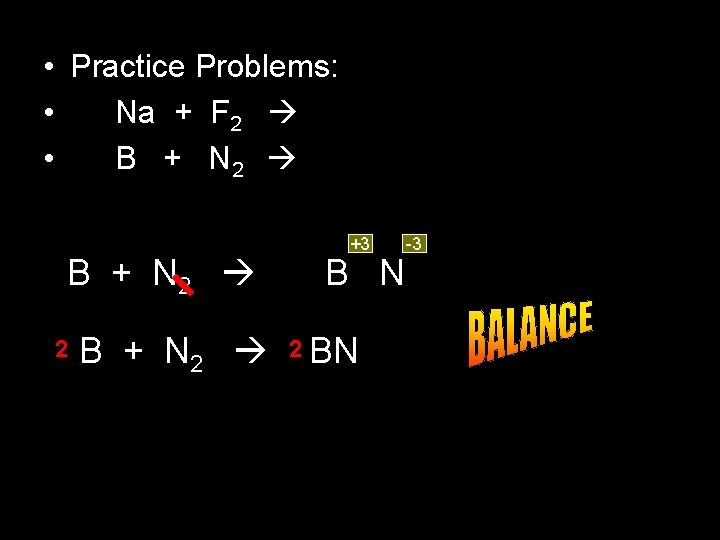
• Practice Problems: • Na + F 2 • B + N 2 2 B + N 2 +3 B N 2 BN -3

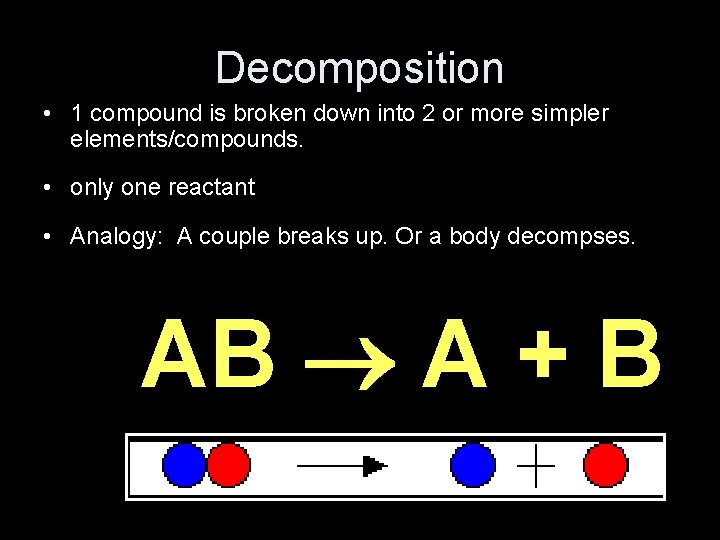
Decomposition • 1 compound is broken down into 2 or more simpler elements/compounds. • only one reactant • Analogy: A couple breaks up. Or a body decompses. AB A + B

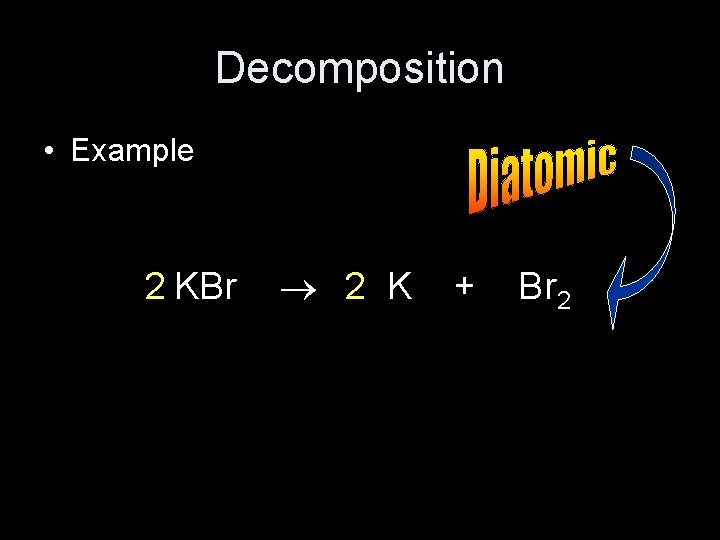
Decomposition • Example 2 KBr 2 K + Br 2

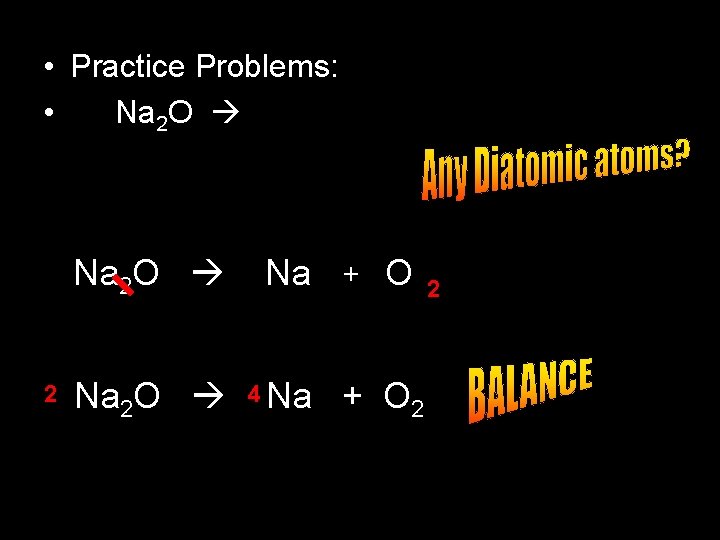
• Practice Problems: • Na 2 O 2 Na 2 O Na Na 2 O 4 Na + O 2 2

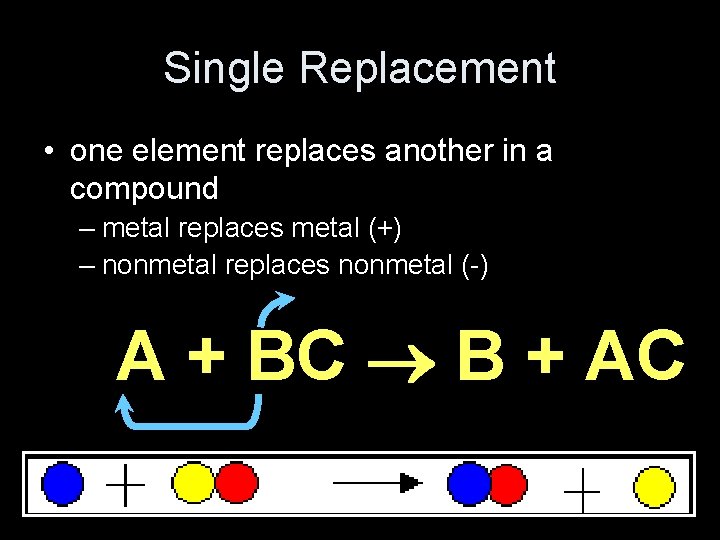
Single Replacement • one element replaces another in a compound – metal replaces metal (+) – nonmetal replaces nonmetal (-) A + BC B + AC

• Products: Metal replacement: 2 Na + Cu. Cl 2 2 Na. Cl + Cu Halogen replacement: F 2 + 2 KCl 2 KF + Cl 2 – free element must be more active (check activity series) Fe(s)+ Cu. SO 4(aq) Cu(s)+ Fe. SO 4(aq) Br 2(l)+ Na. Cl(aq) N. R.

Metals Nonmetals lithium fluorine potassium chlorine calcium bromine sodium iodine magnesium oxygen aluminum nitrogen zinc chromium iron nickel tin lead hydrogen* copper mercury silver platinum gold

Practice • • Can Al replace Li? Can Cu replace Au? Can Br replace I? Can Cl replace F? NO ____ YES ____ NO ____

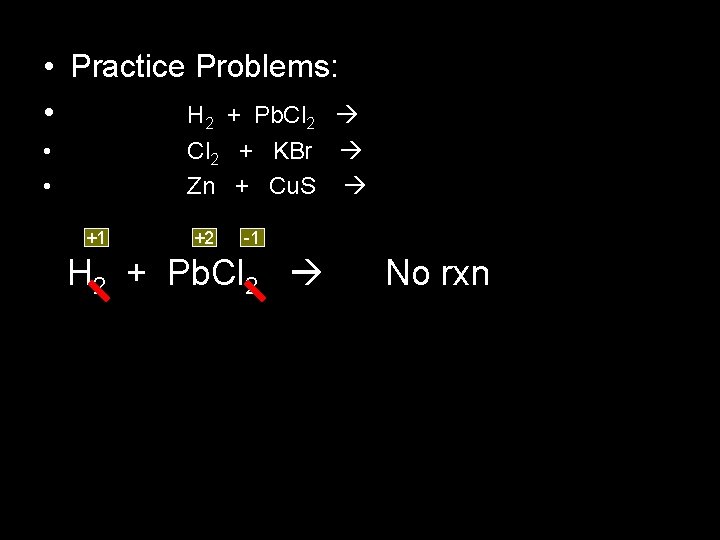
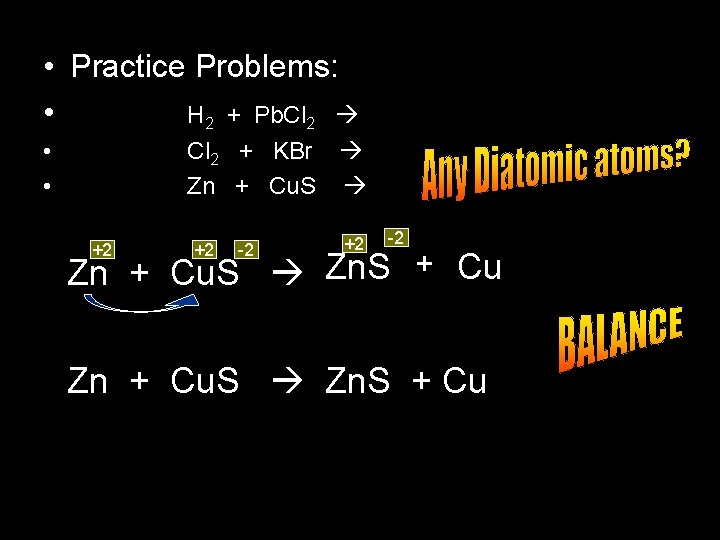
• Practice Problems: • H 2 + Pb. Cl 2 • • Cl 2 + KBr Zn + Cu. S +1 +2 -1 H 2 + Pb. Cl 2 No rxn

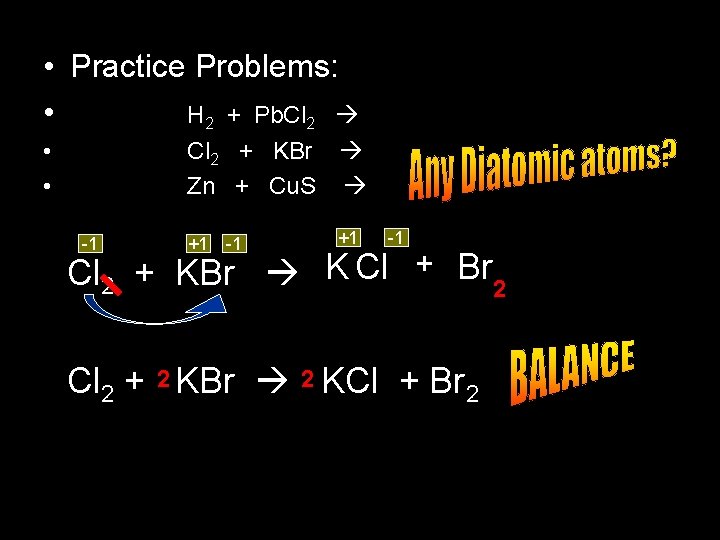
• Practice Problems: • H 2 + Pb. Cl 2 • • Cl 2 + KBr Zn + Cu. S -1 +1 -1 Cl 2 + KBr K Cl + Br 2 Cl 2 + 2 KBr 2 KCl + Br 2

• Practice Problems: • H 2 + Pb. Cl 2 • • Cl 2 + KBr Zn + Cu. S +2 +2 -2 Zn + Cu. S Zn. S + Cu

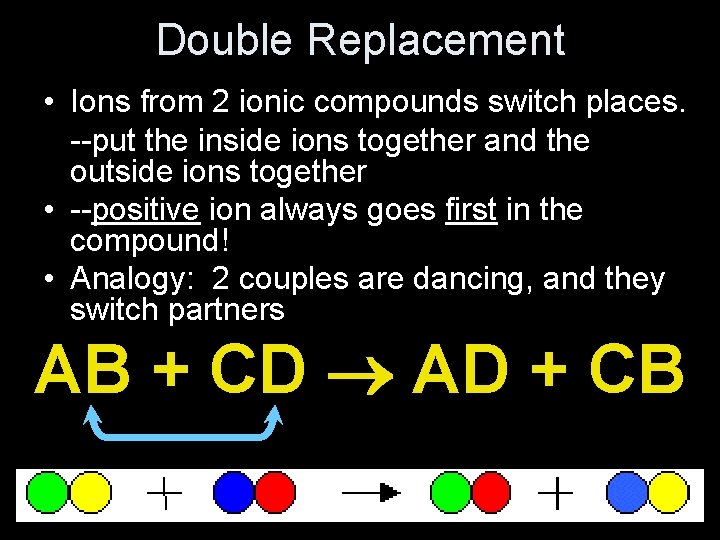
Double Replacement • Ions from 2 ionic compounds switch places. --put the inside ions together and the outside ions together • --positive ion always goes first in the compound! • Analogy: 2 couples are dancing, and they switch partners AB + CD AD + CB

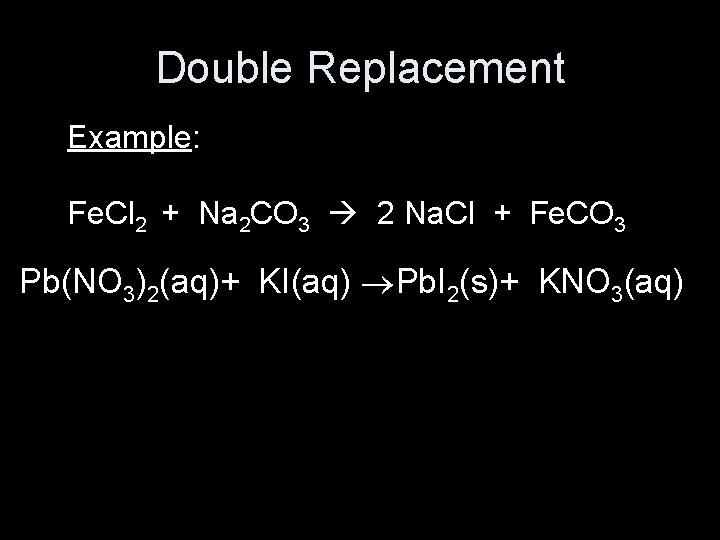
Double Replacement Example: Fe. Cl 2 + Na 2 CO 3 2 Na. Cl + Fe. CO 3 Pb(NO 3)2(aq)+ KI(aq) Pb. I 2(s)+ KNO 3(aq)

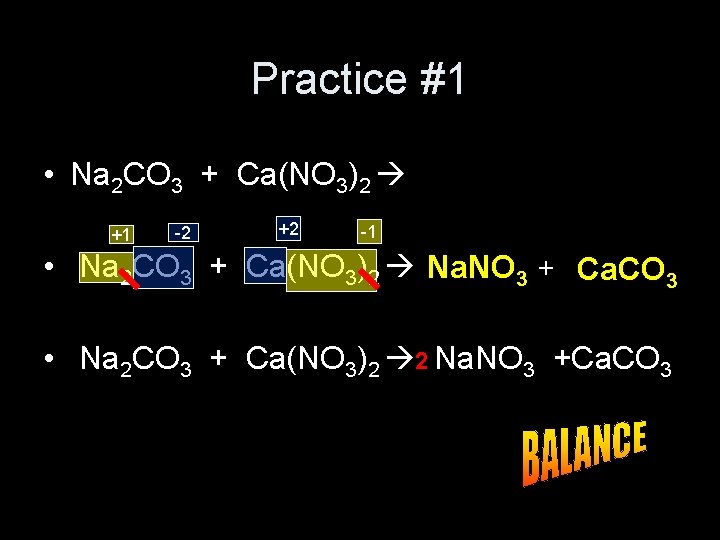
Practice #1 • Na 2 CO 3 + Ca(NO 3)2 +1 -2 +2 -1 • Na 2 CO 3 + Ca(NO 3)2 Na. NO 3 + Ca. CO 3 • Na 2 CO 3 + Ca(NO 3)2 2 Na. NO 3 +Ca. CO 3

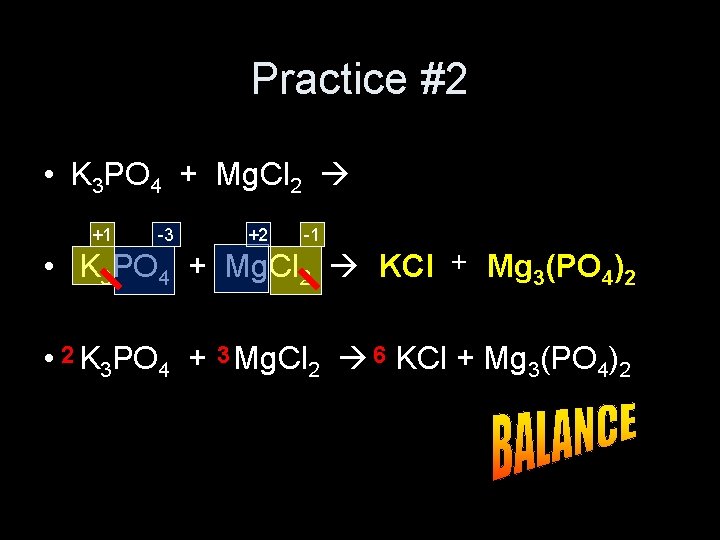
Practice #2 • K 3 PO 4 + Mg. Cl 2 +1 -3 +2 -1 • K 3 PO 4 + Mg. Cl 2 KCl + Mg 3(PO 4)2 • 2 K 3 PO 4 + 3 Mg. Cl 2 6 KCl + Mg 3(PO 4)2

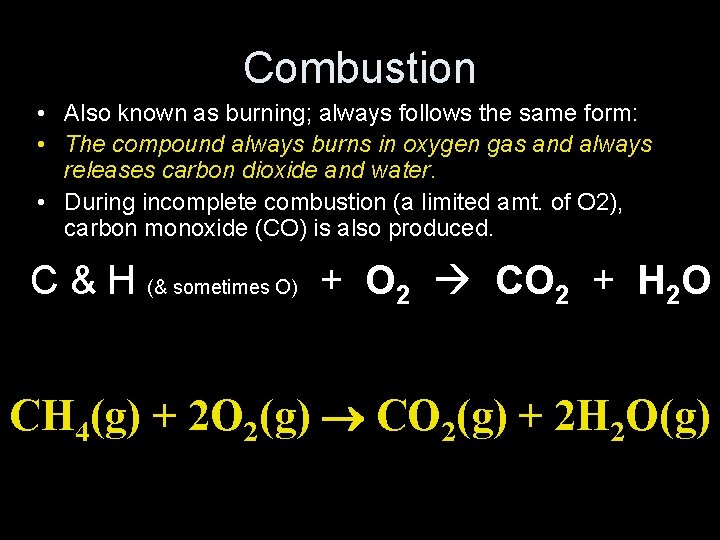
Combustion • Also known as burning; always follows the same form: • The compound always burns in oxygen gas and always releases carbon dioxide and water. • During incomplete combustion (a limited amt. of O 2), carbon monoxide (CO) is also produced. C & H (& sometimes O) + O 2 CO 2 + H 2 O CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)

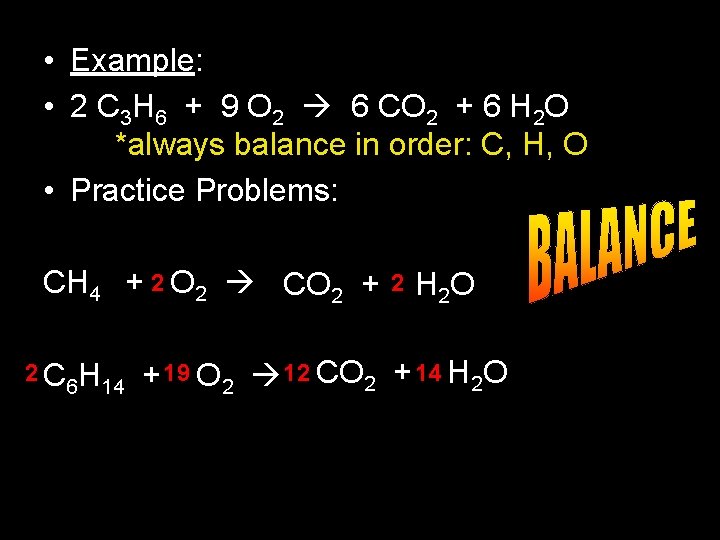
• Example: • 2 C 3 H 6 + 9 O 2 6 CO 2 + 6 H 2 O *always balance in order: C, H, O • Practice Problems: CH 4 + 2 O 2 CO 2 + 2 C 6 H 14 2 H 2 O + 19 O 2 12 CO 2 + 14 H 2 O