Unit 8 Chemical Quantities Information in a Chemical




- Slides: 15

Unit 8 Chemical Quantities

Information in a Chemical Equation Remembe r 1. A chemical equation tells you what reactant chemicals are used and what product chemicals are formed. 2. A chemical equation does tell you the ratio to mix the chemicals in with the coefficients. 3. A chemical equation does not tell you how long the chemical reaction will take, how to mix the chemicals, or at what temperature.

Reactions can be scaled up or down just like a recipe. 2 eggs + 3 cup flour + 1. 5 cup brown sugar + 1 bag chocolate chips + 1 tsp vanilla + 2/ 3 cup butter = 5 doz. cookies

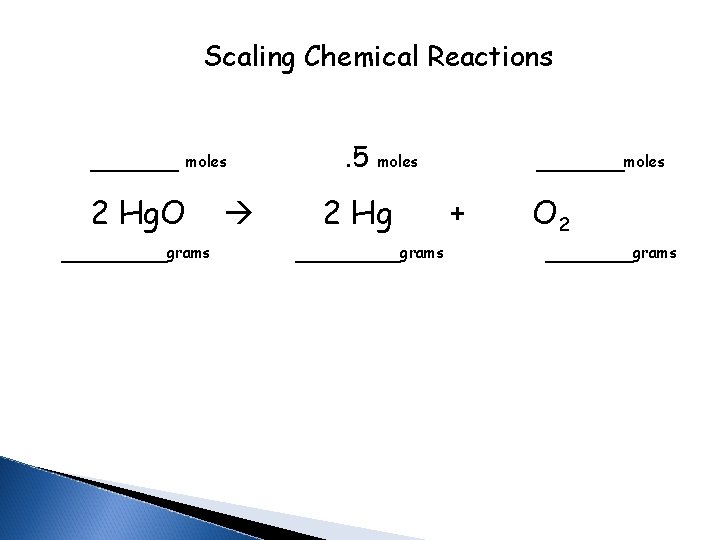
Scaling Chemical Reactions _____ 2 Hg. O moles ______grams . 5 _____moles 2 Hg ______grams + O 2 _____grams

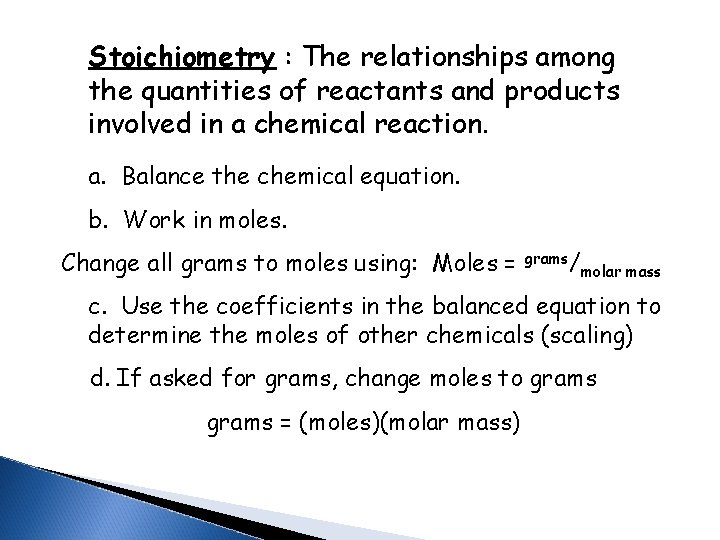
Stoichiometry : The relationships among the quantities of reactants and products involved in a chemical reaction. a. Balance the chemical equation. b. Work in moles. Change all grams to moles using: Moles = grams/ molar mass c. Use the coefficients in the balanced equation to determine the moles of other chemicals (scaling) d. If asked for grams, change moles to grams = (moles)(molar mass)

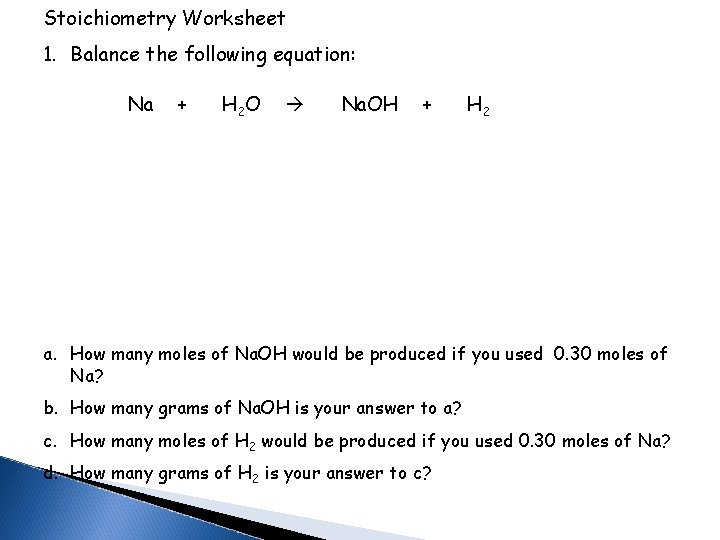
Stoichiometry Worksheet 1. Balance the following equation: Na + H 2 O Na. OH + H 2 a. How many moles of Na. OH would be produced if you used 0. 30 moles of Na? b. How many grams of Na. OH is your answer to a? c. How many moles of H 2 would be produced if you used 0. 30 moles of Na? d. How many grams of H 2 is your answer to c?

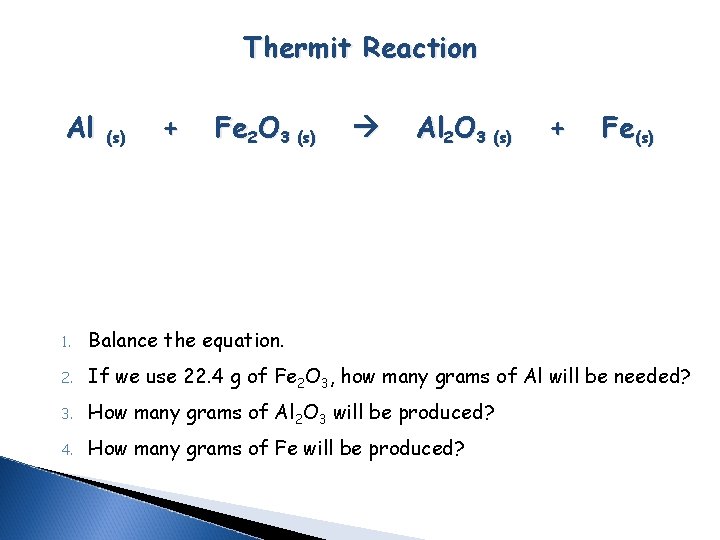
Thermit Reaction Al (s) + Fe 2 O 3 (s) Al 2 O 3 (s) + Fe(s) 1. Balance the equation. 2. If we use 22. 4 g of Fe 2 O 3, how many grams of Al will be needed? 3. How many grams of Al 2 O 3 will be produced? 4. How many grams of Fe will be produced?

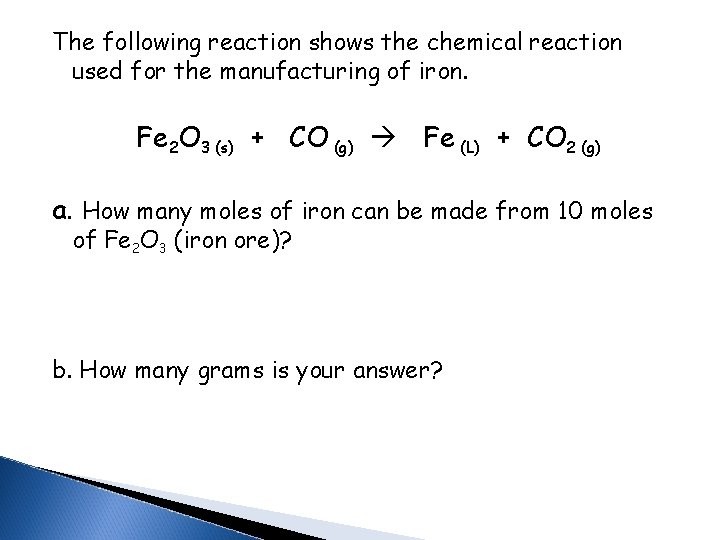
The following reaction shows the chemical reaction used for the manufacturing of iron. Fe 2 O 3 (s) + CO (g) Fe (L) + CO 2 (g) a. How many moles of iron can be made from 10 moles of Fe 2 O 3 (iron ore)? b. How many grams is your answer?

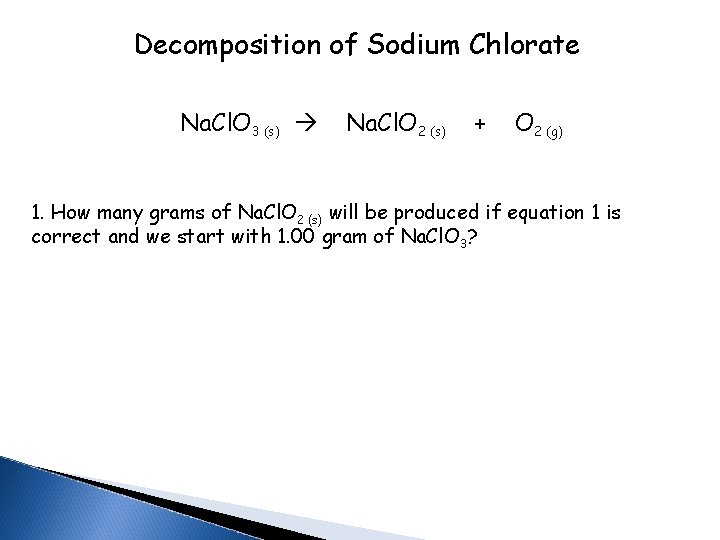
Decomposition of Sodium Chlorate Na. Cl. O 3 (s) Na. Cl. O 2 (s) + O 2 (g) 1. How many grams of Na. Cl. O 2 (s) will be produced if equation 1 is correct and we start with 1. 00 gram of Na. Cl. O 3?

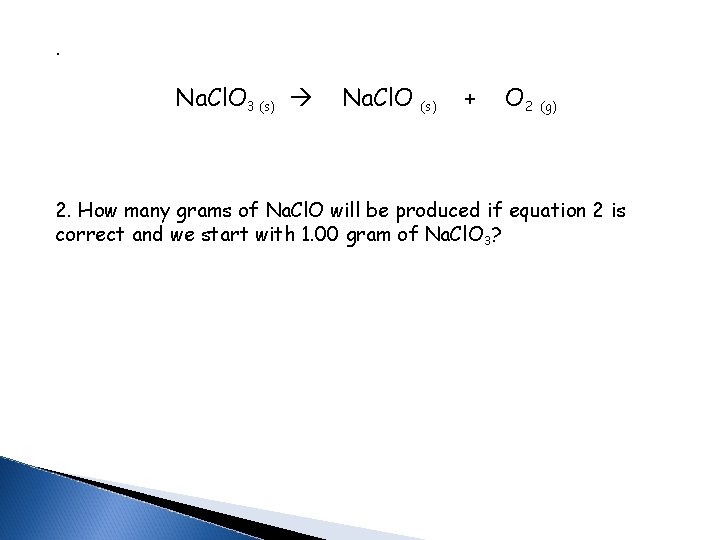
. Na. Cl. O 3 (s) Na. Cl. O (s) + O 2 (g) 2. How many grams of Na. Cl. O will be produced if equation 2 is correct and we start with 1. 00 gram of Na. Cl. O 3?

Na. Cl. O 3 (s) Na. Cl (s) + O 2 (g) 3. How many grams of Na. Cl will be produced if equation 3 is correct and we start with 1. 00 gram of Na. Cl. O 3?

Steps for Solving for Limiting Reactant 1. Write and balance the equation for the reaction 2. Convert know masses to moles for reactants 3. Scale both reactants to one product The smaller amount of the scaling identifies the Limiting reactant 4. Use moles (calculated using the Limiting reactant) to convert to grams of products 5. Scale the moles of the limiting reactant to the excess reactant subtract from the given amount to find excess moles 6. Use the difference in moles to determine excess grams

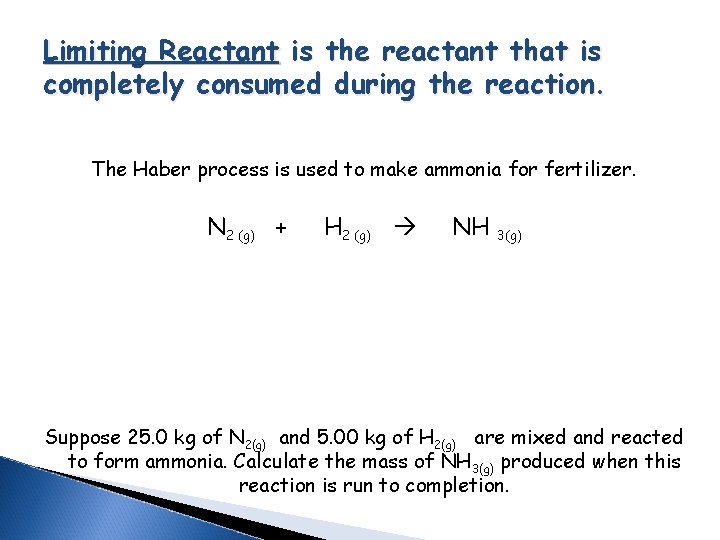
Limiting Reactant is the reactant that is completely consumed during the reaction. The Haber process is used to make ammonia for fertilizer. N 2 (g) + H 2 (g) NH 3(g) Suppose 25. 0 kg of N 2(g) and 5. 00 kg of H 2(g) are mixed and reacted to form ammonia. Calculate the mass of NH 3(g) produced when this reaction is run to completion.

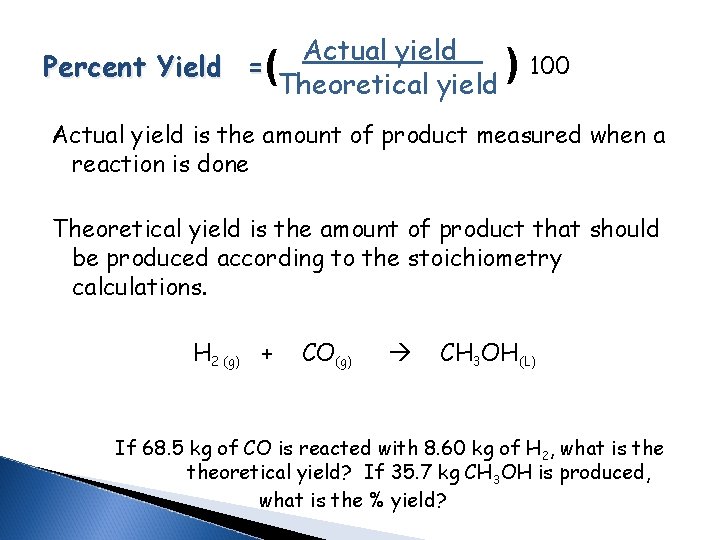
( Actual yield Percent Yield = ( Theoretical yield 100 Actual yield is the amount of product measured when a reaction is done Theoretical yield is the amount of product that should be produced according to the stoichiometry calculations. H 2 (g) + CO(g) CH 3 OH(L) If 68. 5 kg of CO is reacted with 8. 60 kg of H 2, what is theoretical yield? If 35. 7 kg CH 3 OH is produced, what is the % yield?

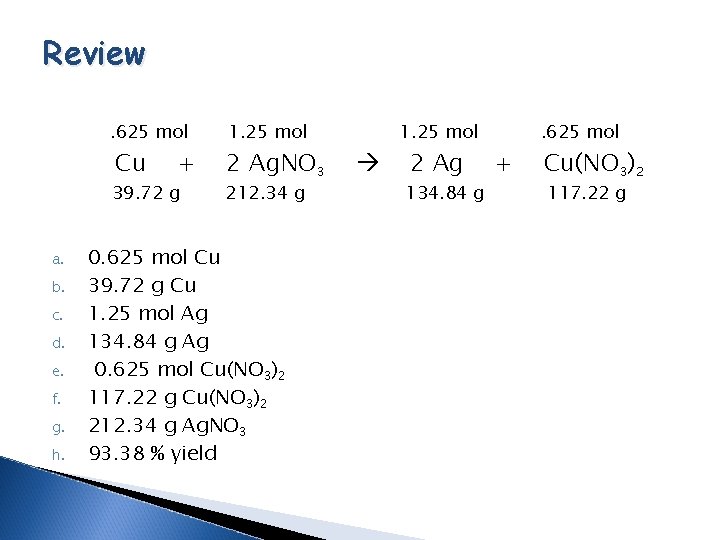
Review. 625 mol Cu + 39. 72 g a. b. c. d. e. f. g. h. 1. 25 mol 2 Ag. NO 3 212. 34 g 0. 625 mol Cu 39. 72 g Cu 1. 25 mol Ag 134. 84 g Ag 0. 625 mol Cu(NO 3)2 117. 22 g Cu(NO 3)2 212. 34 g Ag. NO 3 93. 38 % yield 1. 25 mol 2 Ag 134. 84 g + . 625 mol Cu(NO 3)2 117. 22 g