Unit 8 Bond Type Objectives Remember what is
Unit 8: Bond Type Objectives Remember what is electronegativity. Determine polar vs nonpolar bonds given an electronegativity chart Define terms. Words to know Electronegativity Polarity Covalent Polar Covalent Ionic Cohesion Adhesion Intramolecular vs intermolecular Surface Tension
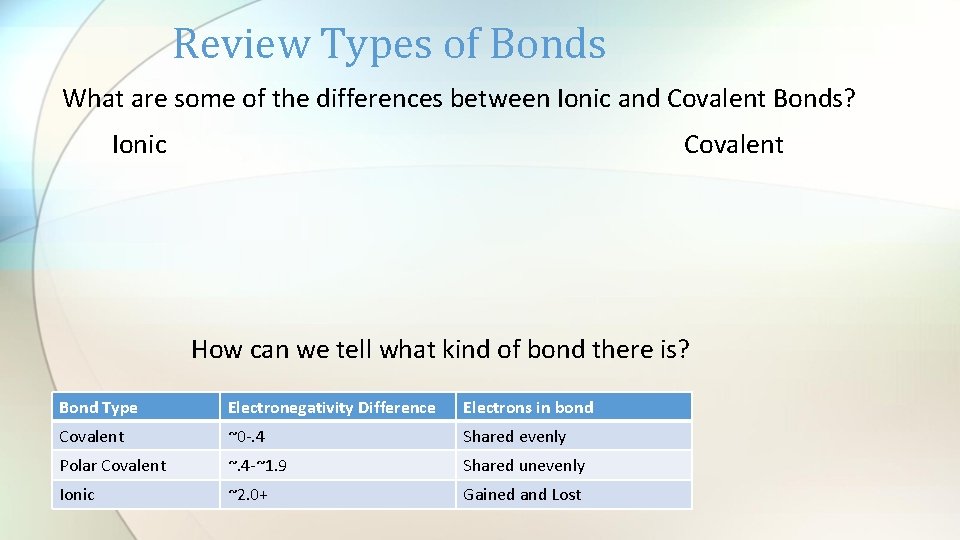
Review Types of Bonds What are some of the differences between Ionic and Covalent Bonds? Ionic Covalent How can we tell what kind of bond there is? Bond Type Electronegativity Difference Electrons in bond Covalent ~0 -. 4 Shared evenly Polar Covalent ~. 4 -~1. 9 Shared unevenly Ionic ~2. 0+ Gained and Lost
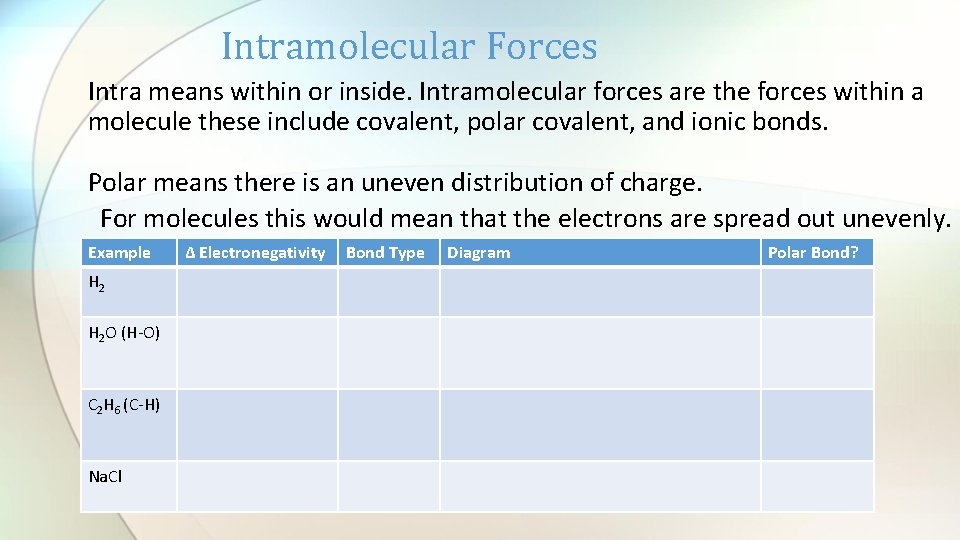
Intramolecular Forces Intra means within or inside. Intramolecular forces are the forces within a molecule these include covalent, polar covalent, and ionic bonds. Polar means there is an uneven distribution of charge. For molecules this would mean that the electrons are spread out unevenly. Example H 2 O (H-O) C 2 H 6 (C-H) Na. Cl ∆ Electronegativity Bond Type Diagram Polar Bond?
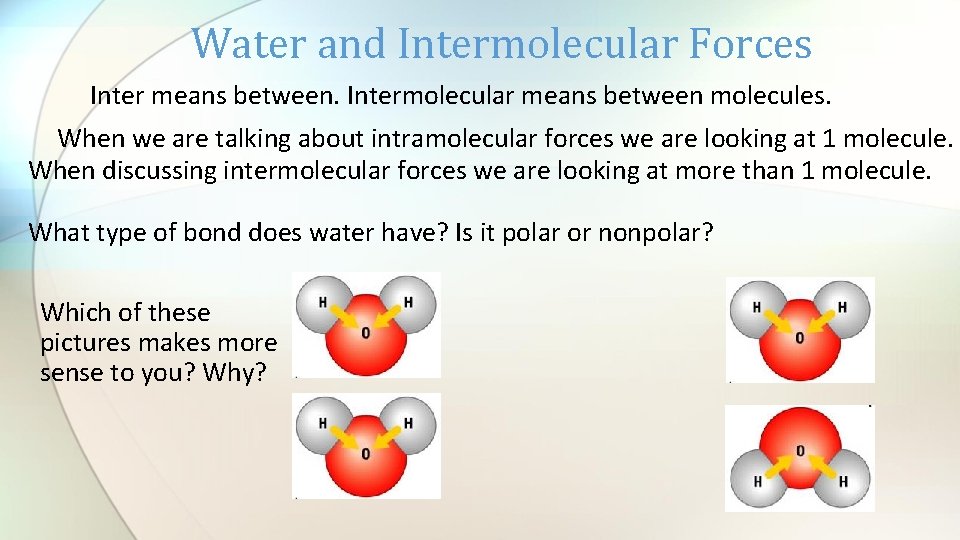
Water and Intermolecular Forces Inter means between. Intermolecular means between molecules. When we are talking about intramolecular forces we are looking at 1 molecule. When discussing intermolecular forces we are looking at more than 1 molecule. What type of bond does water have? Is it polar or nonpolar? Which of these pictures makes more sense to you? Why?
- Slides: 4